- #1
ohms law
- 70
- 0
I figured out the answer to this already, but I wanted help on the reasoning behind it:
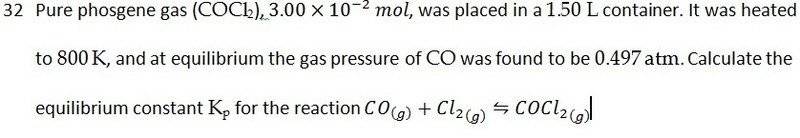
In order to work out the problem we're supposed to determine the partial pressure of phosgene by subtracting 0.497 atm worth from the initial pressure of 1.31 atm (determined by using the ideal gas equation). My question is... why? I mean, if there's 0.497 atm of CO(g) and the stoichiometric ratios of the balanced equation are 1:1:1, then why aren't all three gases at 0.497 atm?
In order to work out the problem we're supposed to determine the partial pressure of phosgene by subtracting 0.497 atm worth from the initial pressure of 1.31 atm (determined by using the ideal gas equation). My question is... why? I mean, if there's 0.497 atm of CO(g) and the stoichiometric ratios of the balanced equation are 1:1:1, then why aren't all three gases at 0.497 atm?
Last edited: