- #1
Biker
- 416
- 52
I have seen some sites say that it doesn't and other says the opposite. So let's see
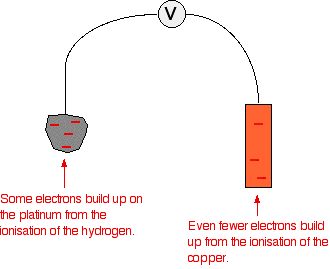
Source of the pic: Chemguide
Lets look at the hydrogen (Oxidation) half cell. If we assume that it contributes x volts until the electron reachs the copper, then no matter how big is the distance the voltage is going to be the same ( only the Electric field will be weak and the current will decrease)
However if we look at both cells together and try to move the copper away from the hydrogen and look at the contribution of the copper electrode, First opposing the current, by exerting a force in the other direction. So when you move it away, you are making the potential of the electrons in the platinum decrease.
So if we assume that the voltage at the copper electrode ( without the contribution of hydrogen) is y and the voltage at the hydrogen is z at the initial state then you move it away z will decrease so the difference will increase thus opposing the current moreIs this a explanation true?
Source of the pic: Chemguide
Lets look at the hydrogen (Oxidation) half cell. If we assume that it contributes x volts until the electron reachs the copper, then no matter how big is the distance the voltage is going to be the same ( only the Electric field will be weak and the current will decrease)
However if we look at both cells together and try to move the copper away from the hydrogen and look at the contribution of the copper electrode, First opposing the current, by exerting a force in the other direction. So when you move it away, you are making the potential of the electrons in the platinum decrease.
So if we assume that the voltage at the copper electrode ( without the contribution of hydrogen) is y and the voltage at the hydrogen is z at the initial state then you move it away z will decrease so the difference will increase thus opposing the current moreIs this a explanation true?