- #1
etotheipi
I've been reading through this paper to try and get a better understanding of how batteries work. The analysis there is fine (they consider a voltaic cell to charge a capacitor in order to derive ##\Delta V=\varepsilon##, and go via an energy route), but it doesn't really touch upon the fields inside the battery. I assume that there are no non-conservative electric fields inside the battery arising from time varying magnetic fields (this could be wrong...).
If I take A to be the negative terminal and B to be the positive terminal, then the EMF from A to B is the line integral of all forces (per unit charge), $$\varepsilon = \int_A^B (\frac{1}{q} \vec{F}_{chemical} + \vec{E}) \cdot d\vec{r}$$ Whilst the change in potential from A to B is $$\Delta V = -\int_A^B \vec{E} \cdot d\vec{r}$$ I am not sure how we can show from here that these two quantities are equal. To make matters worse, Wikipedia says this:
There are quite a few things I don't understand about this. First of all, they use a field ##\mathbf{E}_{cs}## - is that to imply that they are performing a decomposition into a conservative component and non-conservative component ##\mathbf{E}_{ncs}##? And if so, why would the non-conservative component arise?
The only way I can make sense of Wikipedia's definition is to suppose that the electric field can be decomposed ##\vec{E} = \vec{E}_{cs} + \vec{E}_{ncs}##, and then asserting that ##\vec{E}=0## and ##\frac{1}{q}F_{chemical} = -\vec{E}_{cs}##. Though I'm not sure if this makes any sense at all.
So I wondered whether anyone could clear up what is actually going on? Thanks!
If I take A to be the negative terminal and B to be the positive terminal, then the EMF from A to B is the line integral of all forces (per unit charge), $$\varepsilon = \int_A^B (\frac{1}{q} \vec{F}_{chemical} + \vec{E}) \cdot d\vec{r}$$ Whilst the change in potential from A to B is $$\Delta V = -\int_A^B \vec{E} \cdot d\vec{r}$$ I am not sure how we can show from here that these two quantities are equal. To make matters worse, Wikipedia says this:
Inside a source of emf that is open-circuited, the conservative electrostatic field created by separation of charge exactly cancels the forces producing the emf. Thus, the emf has the same value but opposite sign as the integral of the electric field aligned with an internal path between two terminals A and B of a source of emf in open-circuit condition (the path is taken from the negative terminal to the positive terminal to yield a positive emf, indicating work done on the electrons moving in the circuit).[13] Mathematically:
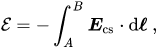
There are quite a few things I don't understand about this. First of all, they use a field ##\mathbf{E}_{cs}## - is that to imply that they are performing a decomposition into a conservative component and non-conservative component ##\mathbf{E}_{ncs}##? And if so, why would the non-conservative component arise?
The only way I can make sense of Wikipedia's definition is to suppose that the electric field can be decomposed ##\vec{E} = \vec{E}_{cs} + \vec{E}_{ncs}##, and then asserting that ##\vec{E}=0## and ##\frac{1}{q}F_{chemical} = -\vec{E}_{cs}##. Though I'm not sure if this makes any sense at all.
So I wondered whether anyone could clear up what is actually going on? Thanks!