- #1
Jens Lundell
- 7
- 0
Newbie here: Is the (single) electron leaving the "machine" in the famous double-slit experiment the same one hitting the screen? Please give a short explanation on how this is proved, thank you.
PeroK said:Electrons are indistiguishable. There is no way to mark, label or number an electron to distinguish it from others. Technically, therefore, all you know is that an electron left the machine and an electron hit the screen. In fact, it would make no sense to ask if it was "the same electron".
Jens Lundell said:Thank you for the fast answer. But isn't this huge, the fact that we don't really know if the electron we fired is the one hitting the screen??
PeroK said:It's not that we don't know (whether it's the same electron), it's that it doesn't make sense to ask the question.
Jens Lundell said:I recognize this type of answer from somewhere. But I'll play along: why does it not make sense to ask the question? :)
PeroK said:It's not a question of playing along. All elementary particles, by virtue of what they are, cannot be changed or marked or numbered in any way. The reason you can identify, say, one pool ball from another is that you can change each ball (paint a number on it) and it's still a pool ball.
Even if you could paint a number on an electron, it would no longer be an electron. If you, say, attach it to a proton to it, then it becomes a hydrogen atom. And all hydrogen atoms are likewise indistinguishable.
All you can ever know about an electon is that it is an electron. You cannot identify it further in any way.
The indistinguishability of electrons is, in fact, at the root of all chemistry, so it is of physically fundamental importance.
Jens Lundell said:Thank you again. I was thinking about the fact that the fired electron takes all possible trajectories simultaneously before hitting the screen. Instead of saying this, could it maybe be that the fired electron is not taking all the trajectories simultaneously, but rather "bumping" all the other electorns in its straight path, where the last bumped electron hit the screen? Something like the pool ball hitting the other ones and another ball ends up in the pocket. I am starting to feel like there is holes to my theory but I'll still post it so maybe you can add/contract from it. :)
Agreed.PeroK said:Electrons are indistiguishable. There is no way to mark, label or number an electron to distinguish it from others. Technically, therefore, all you know is that an electron left the machine and an electron hit the screen.
Not agreed. If electron emission is slowed down so that each electron is individually detected then it makes perfect sense to think it might be the "same" electron as was emitted if we had detected that electron at emission without significantly disturbing its flight. It doesn't guarantee it, of course, because it might have been annihilated and replaced en route.In fact, it would make no sense to ask if it was "the same electron".
PeroK said:What do you mean by the electron takes all possible trajectories simultaneously? Do you think it decides where on the screen it wants to hit first, then gets there by all possible trajectories? Or, if it sets out on all possible trajectories, how does it decide where to hit the screen?
PeroK said:What do you mean by the electron takes all possible trajectories simultaneously? Do you think it decides where on the screen it wants to hit first, then gets there by all possible trajectories? Or, if it sets out on all possible trajectories, how does it decide where to hit the screen?
mikeyork said:Agreed.
Not agreed. If electron emission is slowed down so that each electron is individually detected then it makes perfect sense to think it might be the "same" electron as was emitted if we had detected that electron at emission without significantly disturbing its flight. It doesn't guarantee it, of course, because it might have been annihilated and replaced en route.
So, in the sense of the OP, where he is contrasting the idea of a single electron to multiple collisions and "bumping" electrons, I think we can say quite clearly that their idea would be inconsistent with a tightly constrained momentum state (and interference pattern) and they should definitely prefer the notion of the "same" electron..
I think this illustrates how careful we must be with our language when we stray from mathematics.
The OP's context was an interference experiment which implies a closely defined momentum.mike1000 said:I hope I am not changing the subject too much...but in that experiment is the electrons momentum presumed to be well known, ie, in the preparation state the electrons momentum is placed in a well defined state? (If it is too much off topic I understand if this post is deleted)
Jens Lundell said:I am reading Brian Greene's book "the elegant universe". In there he writes about the Feynmans double-slit experiment and uncertainty principle. Under a figure (which I don't know how to insert, but it is only showing a scematic picture of a standard double-slit experiment) it says:
"According to Feynman's formulation of quantum mechanics, particles must be viewed as
travelling from one location to another along every possible path. Here, a few of the infinity of
trajectories for a single electron traveling from the source to the phosphorescent screen are shown.
Notice that this one electron actually goes through both slits."
So it says that the electorn goes through both slits and every possible path simultaneously before hitting the screen. This is impossible for me to wrap my head around, that it takes every possible path at the same time! So I was thinking maybe it doesn't? That the electron hitting the screen is not the one that left the gun? Again, please keep in mind that I am very new to physics and may have misunderstood the whole thing.
PeroK said:No, you haven't misunderstood. But, Brian Greene is being very imprecise. The only way you can know where an electron is at any time is to measure it. If you don't measure an electron until it hits the screen, then you cannot say how it got there (in terms of a classical trajectory). The Feynman formulation provides a way to calculate the probability of where on the screen the electron hits by considering all possiblities. But, in fact, it's evolution of the electron's wave-function which is considered.
In particular, you can never say an electron went through both slits unless you looked for it at both slits and then you would find it only goes through one.
Jens Lundell said:Okay? So why does he write it like this then? Is it a "popular writing" thing?
Jens Lundell said:Another thing about the uncertainty principle. Could it be that it is uncertain where exactly the electron will hit the screen, because an electron spins around an atom and if it is "to the left" of an atom at the point it hits the screen, then it will hit to the left of an imagimanry centre point on the screen? The center point meaning an imaginary straight line between the gun and screen which ends in a point on the screen where the electro should hit when fired.
PeroK said:Yes, partly because even mention of the wave-function requires more time and effort, so it's easier just to talk about the particle. And, partly, perhaps because it sounds interesting and exciting to think of an electron taking an infinite number of paths simultaneously.
No, nothing like this. You could try watching the Feyman lecture here:
http://www.cornell.edu/video/richard-feynman-messenger-lecture-6-probability-uncertainty-quantum-mechanical-view-nature
PeroK said:In particular, you can never say an electron went through both slits unless you looked for it at both slits and then you would find it only went through one.
Wrong. We just don't know which slit it went through. Uncertainty in QM is expressed as "superpositions". So, without having detectors at the slits, we have what is effectively a superposition of "slit states" imposed on the superposition of impact locations at the screen and this results in additive/cancelling terms in the projections onto impact locations. This looks like "interference" because the addition/cancellation is done before computing the intensity at the screen rather than after.mike1000 said:If we don't look for it at either slit can we say it went through both slits? If I did say that, would I be right or would I be wrong?
IMO you would do better to forget about wave-functions entirely. Think of superpositions instead. You may not understand everything, but you'll be nearer to how physicists think through the math.I think I know the answer to this, and that is the wave function has not collapsed so therefore, the electron did go through both slits. Why doesn't going through the slit(without a detector) collapse the wave function? I think I know the answer to this also...it just doesn't. When we do the experiment, that is what we observe. We have a theory that matches observations and agrees with experiment but does not really tell us a whole lot of what is fundamentally taking place, or if it is telling us what is fundamentally taking place, we don't understand it yet.
The quick response is that of course we know - if there's no detector to interact with, then there's no interaction to decohere the wave function. A good layman-friendly reference would be David Lindley's book "Where does the weirdness go?".mike1000 said:I Why doesn't going through the slit(without a detector) collapse the wave function? I think I know the answer to this also...it just doesn't.
I don't think that is quite right. I think it's another example of the problem of translating math into physics. It seems clear to me that the electron interacts with the slit -- it produces (single slit) electron diffraction for instance and this one reason why the observer/collapse scenario is problematic. The difference a detector makes is that the information is recorded. One of the abiding principles of physics that seems to hold up in a QM context is that nature doesn't seek to deceive us. Once information is available and recorded so that it can be subsequently read then any future observations must be consistent with that unless the information is lost (e.g. by a quantum eraser).Nugatory said:if there's no detector to interact with, then there's no interaction
Jens Lundell said:I am reading Brian Greene's book "the elegant universe".
Would Feynman' QED be considered an acceptable source?PeterDonis said:This is a pop science book and is not an acceptable source. You need to look at actual textbooks or peer-reviewed papers. IIRC the Feynman Lectures on Physics (which are now available for free online at Caltech's website) have a good treatment of this experiment.
edguy99 said:Sometimes it helps to view an actual experiment.
http://iopscience.iop.org/article/10.1088/1367-2630/15/3/033018/meta
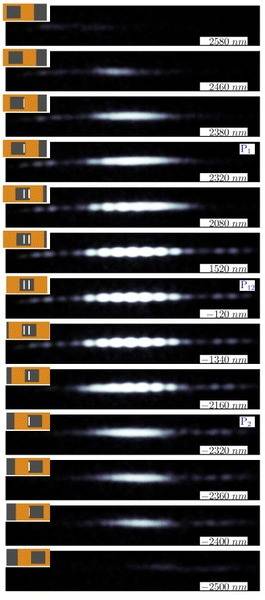
You can read how they prepare and measure the electrons. I especially like this picture. The top left corner shows how much of the double slit is exposed compared to what type of pattern is seen. You can see the diffraction pattern even when there is only one slit open (picture marked P1 or P2) compared to when both are open (pciture marked P12).
Comeback City said:Would Feynman' QED be considered an acceptable source?
This was the one I was referring to.PeterDonis said:QED: The Strange Theory of Light and Matter
I would phrase that in that it depends which information is taken into account. A (delayed) quantum eraser can output an interference pattern simultaneously with a non-interfering pattern. It depends on which data you use.mikeyork said:I don't think that is quite right. I think it's another example of the problem of translating math into physics. It seems clear to me that the electron interacts with the slit -- it produces (single slit) electron diffraction for instance and this one reason why the observer/collapse scenario is problematic. The difference a detector makes is that the information is recorded. One of the abiding principles of physics that seems to hold up in a QM context is that nature doesn't seek to deceive us. Once information is available and recorded so that it can be subsequently read then any future observations must be consistent with that unless the information is lost (e.g. by a quantum eraser).
But it doesn't depend on what data a human uses, it depends on what data is recorded by the apparatus, hasn't been erased and is relevant at the screen. Think of it as the detector and/or eraser as modifying the prepared state that is subsequently detected at the screen.entropy1 said:I would phrase that in that it depends which information is taken into account. A (delayed) quantum eraser can output an interference pattern simultaneously with a non-interfering pattern. It depends on which data you use.
mike1000 said:If we don't look for it at either slit can we say it went through both slits? If I did say that, would I be right or would I be wrong?
mike1000 said:If they would have put detectors at each slit would the diffraction patterns have been observed on the detection screen?
mike1000 said:Also, is the observed scatter of electron positions on the detection screen caused by the uncertainty in the electrons momentum which was inducted when the experiment attempted to localize the particle when it went through the slit?
That is not in any meaningful sense a "reproduction" of the quantum-mechanical double-slit. It's a completely different physical system governed by completely different physical laws and that happens to display interestingly similar behavior so is interesting as an analogy. Considering the ubiquity of the wave equation in physics, it is not surprising that such analogies exist - but an analogy is never the real thing. Thus, you may feel a high degree of confidence, but you shouldn't expect others to share it.ueit said:I think we can say at this time, with a high degree of confidence, that in a double slit experiment the electron passes through one slit. This experiment has been reproduced at macroscopic scale by Yves Couder and Emmanuel Fort.
"Justification" is also much too strong of a claim. There's nothing wrong with Grössing's suggestion that quantum mechanics might emerge from a realistic non-local hidden variable theory of the sort that he is considering (although without a candidate theory there's only so far it can be taken), and Couder's analogy suggests one picture of what such a theory might look like. But that's not a theoretical justification, it's an idea about one possible area of exploration.The relevance of Couder's experiment to quantum mechanics is theoretically justified here:
Nugatory said:That is not in any meaningful sense a "reproduction" of the quantum-mechanical double-slit. It's a completely different physical system governed by completely different physical laws and that happens to display interestingly similar behavior so is interesting as an analogy. Considering the ubiquity of the wave equation in physics, it is not surprising that such analogies exist - but an analogy is never the real thing. Thus, you may feel a high degree of confidence, but you shouldn't expect others to share it.
"Justification" is also much too strong of a claim. There's nothing wrong with Grössing's suggestion that quantum mechanics might emerge from a realistic non-local hidden variable theory of the sort that he is considering (although without a candidate theory there's only so far it can be taken), and Couder's analogy suggests one picture of what such a theory might look like. But that's not a theoretical justification, it's an idea about one possible area of exploration.
The double-slit experiment is a classic experiment in quantum mechanics that demonstrates the wave-particle duality of light and electrons. It involves passing a beam of particles (such as electrons) through two narrow slits and observing the resulting interference pattern on a screen.
In the double-slit experiment, a beam of particles (such as electrons) is directed towards a barrier with two narrow slits. The particles then pass through the slits and create an interference pattern on a screen placed behind the barrier. This pattern is a result of the particles behaving like waves and interfering with each other.
The double-slit experiment is significant because it demonstrates the wave-particle duality of matter. It shows that particles, such as electrons, can exhibit both wave-like and particle-like behavior depending on how they are observed or measured.
In the double-slit experiment, the electron is the particle that is being passed through the two slits. It is used to demonstrate the wave-particle duality of matter and the interference patterns that can occur when particles behave like waves.
The double-slit experiment is a fundamental experiment in quantum mechanics. It shows that particles, such as electrons, can exhibit wave-like behavior and that their behavior is influenced by the act of observation. This experiment played a significant role in the development of quantum mechanics and our understanding of the behavior of matter at the subatomic level.