- #1
Iwanttolearnphysics
- 44
- 9
- Homework Statement
- Calculate the enthalpy change of neutralization for the following reaction
- Relevant Equations
- q = mcΔT
Hi, everyone! There's a question I found on website I'm using and the answer key here is given. My question is this, where did the 400g come from?
According to the definition of enthalpy of neutralization (chem libretexts), the standard enthalpy change of neutralization is the enthalpy change when solutions of an acid and an alkali react together under standard conditions to produce 1 mole of water.
I highlighted 1 mole of water because that's what I used to solve the problem.
This is what I did step by step:
What did I do wrong? Thank you.
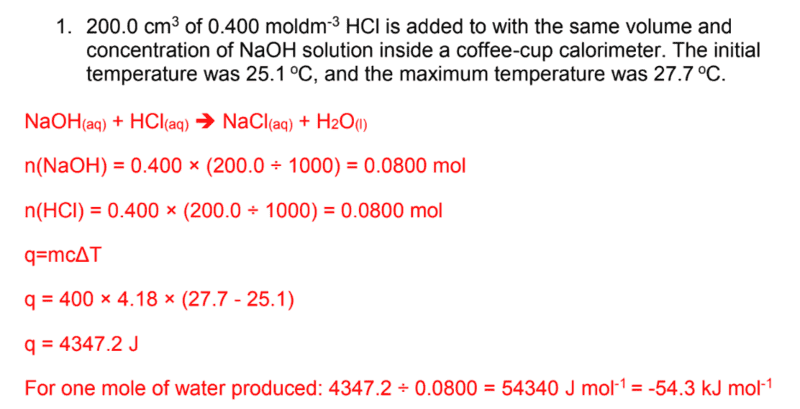
According to the definition of enthalpy of neutralization (chem libretexts), the standard enthalpy change of neutralization is the enthalpy change when solutions of an acid and an alkali react together under standard conditions to produce 1 mole of water.
I highlighted 1 mole of water because that's what I used to solve the problem.
This is what I did step by step:
- First, I wrote the balanced chemical equation. I know that 0.800 mol of NaOH and 0.800 mol of HCl was consumed. From the balanced chemical equation, I concluded that 0.800 mol of water must have been produced too.
- 0.800 mol of water is equivalent to 14.4g of water.
- I used q = mcΔT and I wrote:
- q = 14.4g x 4.18 x (27.7-25.1)
- q = 156.50 J of heat was released per 0.800 mol
- If I want to get per mol, then 156.50J/0.800 mol = 195.62 J/mol
What did I do wrong? Thank you.