- #1
Lo.Lee.Ta.
- 217
- 0
I have been looking at this enzyme kinetics stuff forever and was trying to come up with a summary for things... #=_=
Is this even right...? :/
|---------------|This segment I'm calling "Part 2"
k1 k2
E + S
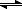 ES
ES
 E + P
E + P
k-1
|---------------|This segment I'm calling "Part 1"
Part 1 and Part 2 are separate reactions, but together, they form the overall enzyme reaction.
Part 1 is dependent on the Km value, Part 2 is dependent on the Kcat value, and the entire enzyme reaction is dependent on the ratio of Kcat to Km (or Kcat/Km).
Part 1:
Since it is dependent on the Km value, and Km = (k-1 + k2)/k1, which means
(ES breakdown)/(ES formation), you have to ask, "Is the ES breakdown higher than the formation?"
If yes, then it means
 Km, so ES tends to break down (slow).
Km, so ES tends to break down (slow).
If no,
 Km, so ES tends to stay together (fast rxn).
Km, so ES tends to stay together (fast rxn).
Part 2:
Have to ask, "Is Kcat high or low?"
If high, fast product formation.
If low, slow product formation.So for the entire enzyme-catalyzed reaction, it seems like there would only be these 4 possibilities:
1.
 Kcat +
Kcat +
 Km = slow for ES to form, but once it does, quickly generates product.2.
Km = slow for ES to form, but once it does, quickly generates product.2.
 Kcat +
Kcat +
 Km = fast for ES to form and quickly generates product.3.
Km = fast for ES to form and quickly generates product.3.
 Kcat +
Kcat +
 Km = slow for ES to form, and once it does, slowly generates product.4.
Km = slow for ES to form, and once it does, slowly generates product.4.
 Kcat +
Kcat +
 Km = fast for ES to form, but slowly generates product.
Km = fast for ES to form, but slowly generates product.
...Well, is any of this even right?

Please help me! D:
Thank you SO much!

Is this even right...? :/
|---------------|This segment I'm calling "Part 2"
k1 k2
E + S
k-1
|---------------|This segment I'm calling "Part 1"
Part 1 and Part 2 are separate reactions, but together, they form the overall enzyme reaction.
Part 1 is dependent on the Km value, Part 2 is dependent on the Kcat value, and the entire enzyme reaction is dependent on the ratio of Kcat to Km (or Kcat/Km).
Part 1:
Since it is dependent on the Km value, and Km = (k-1 + k2)/k1, which means
(ES breakdown)/(ES formation), you have to ask, "Is the ES breakdown higher than the formation?"
If yes, then it means
If no,
Part 2:
Have to ask, "Is Kcat high or low?"
If high, fast product formation.
If low, slow product formation.So for the entire enzyme-catalyzed reaction, it seems like there would only be these 4 possibilities:
1.
...Well, is any of this even right?
Please help me! D:
Thank you SO much!
Last edited by a moderator:

 .
.