- #1
Vivek98phyboy
- 34
- 5
While studying about the effects of surface tension i came across the excess pressure inside a liquid drop.
Here they considered a hemisphere ABCDE from the drop and listed out the conditions for it to be in equilibrium.
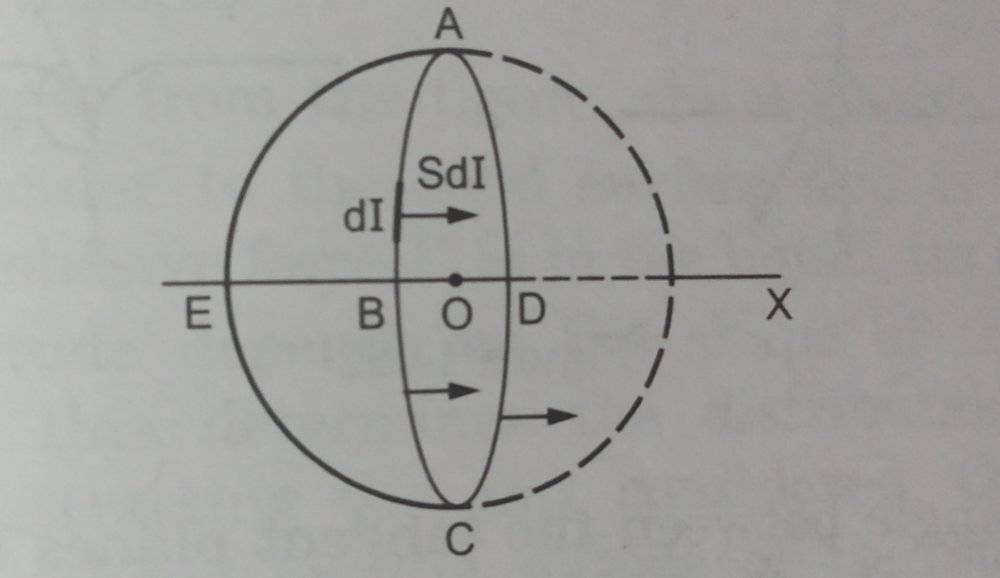
The forces acting on them are taken as

F1= 2πRS
F2= P1×(Projection of hemispherical surface on ABCD)
=>F2=P1×πR²
F3=P2×πR²
For equilibrium we take
F1+F2=F3
But what role does the surface tension (T) has in maintaining the equilibrium for a hemisphere.
My doubts are:
1. Isn't the pressure due to Atmosphere and the pressure inside the hemispherical drop enough to balance each other. Why do we need a surface tension here?
2. When i referred some other sources, it said that the surface tension holds the drop from bursting. If it is so, the force due to T we calculates here acts only along the base periphery of the hemisphere. What effect would it have on the curved surface?
Hope this won't come under Homework help
Here they considered a hemisphere ABCDE from the drop and listed out the conditions for it to be in equilibrium.
The forces acting on them are taken as
F1= 2πRS
F2= P1×(Projection of hemispherical surface on ABCD)
=>F2=P1×πR²
F3=P2×πR²
For equilibrium we take
F1+F2=F3
But what role does the surface tension (T) has in maintaining the equilibrium for a hemisphere.
My doubts are:
1. Isn't the pressure due to Atmosphere and the pressure inside the hemispherical drop enough to balance each other. Why do we need a surface tension here?
2. When i referred some other sources, it said that the surface tension holds the drop from bursting. If it is so, the force due to T we calculates here acts only along the base periphery of the hemisphere. What effect would it have on the curved surface?
Hope this won't come under Homework help