- #1
PainterGuy
- 940
- 69
Hi,
Could you please help me to clarify a few points to understand entropy intuitively?
Entropy is defined as:
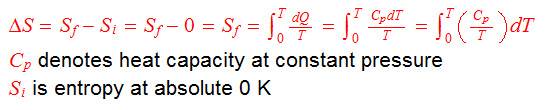
Please have a look at the attachment, "entropy111".
Source of attachment: http://faculty.chem.queensu.ca/people/faculty/mombourquette/chem221/4_secondthirdlaws/SecondLaw.asp
The attachment shows how entropy is calculated for a substance. Figure 1 shows the plot of Cp/T against T and Figure 2 shows the plot of integral ∫(Cp/T)dt from 0 K to 'T' K, i.e. ΔS, entropy. I would say that the plots shown relate closely to those of many real substances.
Question 1:
In Figure 1, the heat capacity, Cp, is less at 'A' compared to at point 'B', while the value of Cp at points 'B' and 'C' is almost equal.
i: Why does Cp become almost constant between 'B' and 'C'?
ii: Between Tf and Tb or between 'D' and 'E', Cp decreases. What could be the reason for this?
iii: Between Tb and T, Cp again decreases drastically. It simply means that less energy is required to raise the temperature by 1 K.
Question 2:
In Figure 2, the entropy is changing at a faster rate at point 'G' than at point 'H'. Do I have it correct?
Question 3:
I have tried to find the entropy of air at 25 °C and 1 atm without any success. The closest I could get was "Entropy of air at 0°C and 1 bar: 0.1100 kJ/mol K = 3.796 kJ/kg K". Could you please help me with it?
Thank you!
Could you please help me to clarify a few points to understand entropy intuitively?
Entropy is defined as:
Please have a look at the attachment, "entropy111".
Source of attachment: http://faculty.chem.queensu.ca/people/faculty/mombourquette/chem221/4_secondthirdlaws/SecondLaw.asp
The attachment shows how entropy is calculated for a substance. Figure 1 shows the plot of Cp/T against T and Figure 2 shows the plot of integral ∫(Cp/T)dt from 0 K to 'T' K, i.e. ΔS, entropy. I would say that the plots shown relate closely to those of many real substances.
Question 1:
In Figure 1, the heat capacity, Cp, is less at 'A' compared to at point 'B', while the value of Cp at points 'B' and 'C' is almost equal.
i: Why does Cp become almost constant between 'B' and 'C'?
ii: Between Tf and Tb or between 'D' and 'E', Cp decreases. What could be the reason for this?
iii: Between Tb and T, Cp again decreases drastically. It simply means that less energy is required to raise the temperature by 1 K.
Question 2:
In Figure 2, the entropy is changing at a faster rate at point 'G' than at point 'H'. Do I have it correct?
Question 3:
I have tried to find the entropy of air at 25 °C and 1 atm without any success. The closest I could get was "Entropy of air at 0°C and 1 bar: 0.1100 kJ/mol K = 3.796 kJ/kg K". Could you please help me with it?
Thank you!





