- #1
fluidistic
Gold Member
- 3,923
- 261
Homework Statement
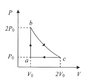
Consider an ideal gas whose volume is initially [tex]V_0=1cm^3[/tex] that is initially at [tex]P_0=1[/tex] atmosphere. It goes through a cycle a-b-c-a where b-c is at constant temperature.
1)According to the sketch, does the system absorb or release heat?
2)Calculate the heat exchange in a complete cycle.
3)What's the total variation of entropy in a complete cycle?
Homework Equations
None given.
The Attempt at a Solution
Since it's an ideal gas, PV=nRT holds.
1)So when the system goes through a-b, its temperature duplicates and as [tex]Q=mc\Delta T[/tex], we have [tex]Q>0[/tex].
For b-c Q=0 since it's an isothermal.
For c-a, heat is released I believe, in the same amount heat has been gained from the environment for the part a-b.
2)Q=0.
3)[tex]\Delta S=\int \frac{dQ}{T}=0[/tex] but I'm unsure.