- #1
TeslaPow
- 40
- 1
I want to calculate the kinetic energy distribution amongst let's say nitrogen molecules by using M.K.E.D, but not sure where to start.
I posted a picturefrom my physics book where the formula is shown, there was no example in the book.
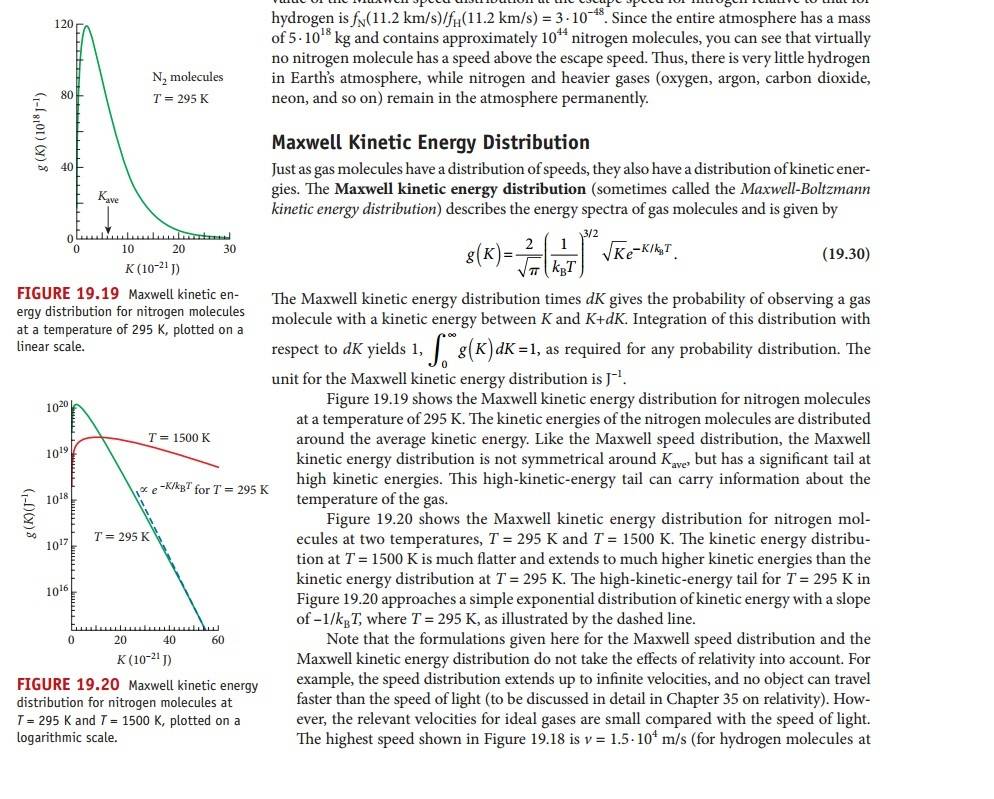
As for g(K), is K the same as the kinetic energy formula Ktot = N[1/2m(v`^2)] = 3/2NKbT or 3/2KbT ?
I posted a picturefrom my physics book where the formula is shown, there was no example in the book.
As for g(K), is K the same as the kinetic energy formula Ktot = N[1/2m(v`^2)] = 3/2NKbT or 3/2KbT ?