- #1
georg gill
- 153
- 6
- TL;DR Summary
- an electron and a magnet at rest sends out B and E lines. How does theese E and B lines look when they come from the moving lone hydrogen electron in the 1s orbital?
Does anyone know theory about how the perturbation lines are for 1s hydrogen electron? By perturbation I mean the perturbation that is caused by moving an electron so that the E-field lines it emits becomes dragged.
by perturbation I mean for example dragging a charge as described below
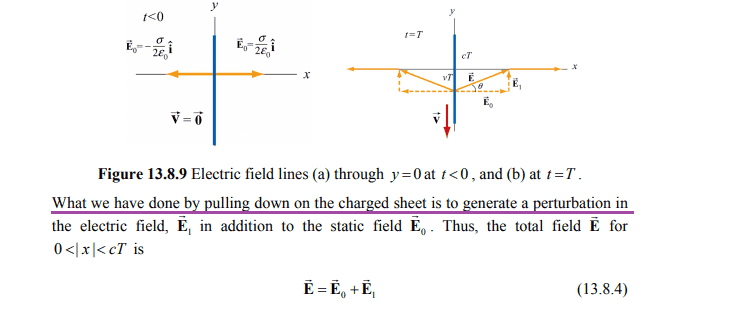
Above is classical theory I assume. I am looking for a classical theory about this or quantum. If there is no perturbation in quantum theory I guess I could ask how does the E and B field lines emitted from an electron from a quantum adressing look like as the electron is moving with its speed 2200 km/s in the 1s orbital compared to how the B and E field look like for a non moving electron from a quantum perspective? You don't have to answer directly if you don't want to as I am primarily looking for theory about this. If it is easier I am also interested in how much energy that is given out from a moving 1s electron compared to an electron at rest when you look at the electromagnetic energy created.
Again I would be thrilled if someone could point out a book about this theory as it could be a bit of a long answer if I would guess myself.
by perturbation I mean for example dragging a charge as described below
Above is classical theory I assume. I am looking for a classical theory about this or quantum. If there is no perturbation in quantum theory I guess I could ask how does the E and B field lines emitted from an electron from a quantum adressing look like as the electron is moving with its speed 2200 km/s in the 1s orbital compared to how the B and E field look like for a non moving electron from a quantum perspective? You don't have to answer directly if you don't want to as I am primarily looking for theory about this. If it is easier I am also interested in how much energy that is given out from a moving 1s electron compared to an electron at rest when you look at the electromagnetic energy created.
Again I would be thrilled if someone could point out a book about this theory as it could be a bit of a long answer if I would guess myself.
Last edited: