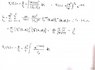- #1
Manuel Galdon
- 4
- 0
EDIT: moved from technical forum, so no template
Hello, I have a problen which is about calculating an electrostatic potential for a hydrogenic atom in the ground state given its wavefunction. Since I know the wavefunction of the ground state I would find it by solving the Schrödinger equation, but the statement gives us an expression to calculate it.
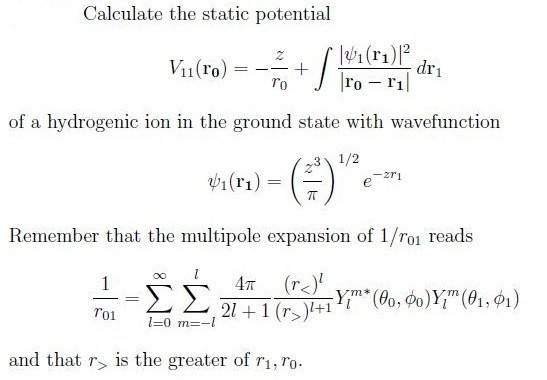
This is all what the problem says. Any ideas? Just put the wavefunction into the integral and go on ?
Thanks !
Hello, I have a problen which is about calculating an electrostatic potential for a hydrogenic atom in the ground state given its wavefunction. Since I know the wavefunction of the ground state I would find it by solving the Schrödinger equation, but the statement gives us an expression to calculate it.
This is all what the problem says. Any ideas? Just put the wavefunction into the integral and go on ?
Thanks !
Last edited by a moderator: