- #1
Jhon81
- 12
- 0
Hi everyone,
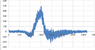
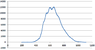
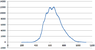
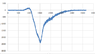
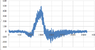
I have an absorption spectrum that I obtained from a sample after radiation and I need to use the background sample as a reference, how can I do that using excel?
what I did is subtract the radiated from the background spectrum then plot it, is that right?
Thanks.
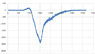
I have an absorption spectrum that I obtained from a sample after radiation and I need to use the background sample as a reference, how can I do that using excel?
what I did is subtract the radiated from the background spectrum then plot it, is that right?
Thanks.