- #1
etotheipi
I came across this diagram, the ##\gamma##'s are supposedly forces per unit length of the respective interfaces:
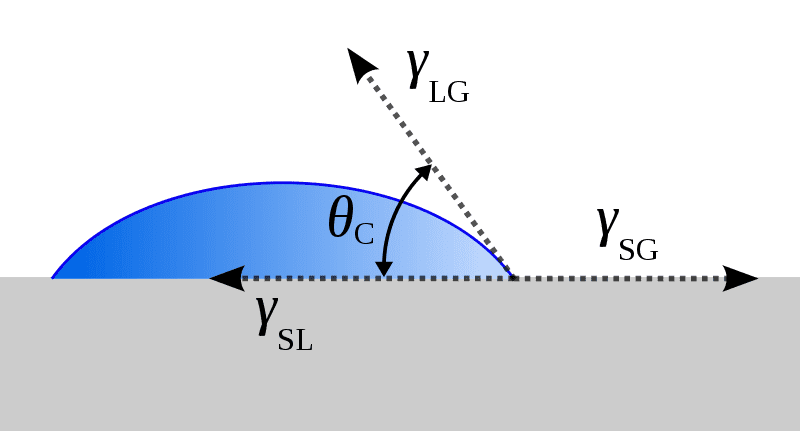
It's not clear what these forces are acting on. ##\gamma_{SL}## and ##\gamma_{LG}## look like they could be acting on a small bit of water right at the end, but I have no idea what ##\gamma_{SG}## is supposed to be acting on.
Likewise there's this diagram of capillary action:
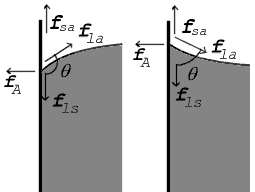
It's not clear here either what bodies any of those forces are acting on.
I wondered if someone could clarify? Thanks
It's not clear what these forces are acting on. ##\gamma_{SL}## and ##\gamma_{LG}## look like they could be acting on a small bit of water right at the end, but I have no idea what ##\gamma_{SG}## is supposed to be acting on.
Likewise there's this diagram of capillary action:
It's not clear here either what bodies any of those forces are acting on.
I wondered if someone could clarify? Thanks