- #1
etotheipi
I will refer to this paper for some relevant bits of theory.
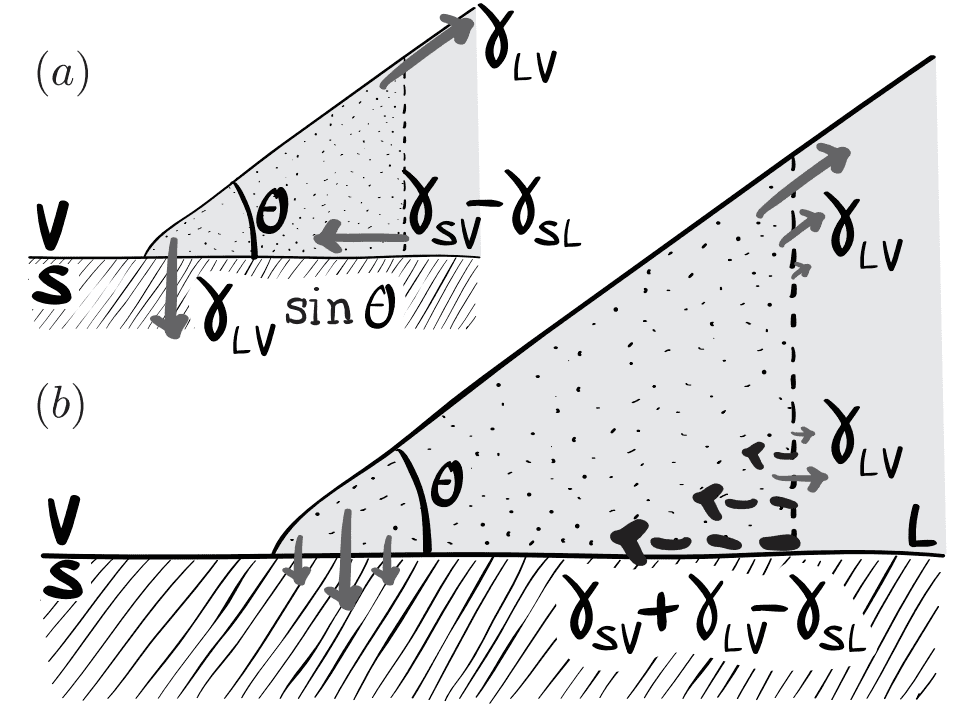
They write that the component of the force between the solid and liquid parallel to the solid-liquid interface, which appears near the contact line (in the dotted region), is ##\gamma_{SV} + \gamma_{LV} - \gamma_{SL}##, which by Young's law also equals ##\gamma_{LV}(1+\cos{\theta})##.
Using the principle of virtual work and some thermodynamic ideas about free energy, we can show that the total force by the whole fluid on the solid parallel to the solid, per unit length of the contour of contact, is ##\gamma_{LV}\cos{\theta}##. The authors also find this result with a mechanical argument,
So there is an additional "Laplace force" caused by the curvature of the plate that has a vertical component per unit length of ##\gamma_{LV}##, which then yields the correct well known result that the surface tension causes an downward component of ##\gamma_{LV}(1+\cos{\theta}) - \gamma_{LV} = \gamma_{LV}\cos{\theta}##.
This is easier to imagine when the partially submerged plate has a lower face exposed to the liquid, but in the case of a liquid in e.g. a beaker, from where does the Laplace force arise?
Thanks!
They write that the component of the force between the solid and liquid parallel to the solid-liquid interface, which appears near the contact line (in the dotted region), is ##\gamma_{SV} + \gamma_{LV} - \gamma_{SL}##, which by Young's law also equals ##\gamma_{LV}(1+\cos{\theta})##.
Using the principle of virtual work and some thermodynamic ideas about free energy, we can show that the total force by the whole fluid on the solid parallel to the solid, per unit length of the contour of contact, is ##\gamma_{LV}\cos{\theta}##. The authors also find this result with a mechanical argument,
The same principle applies to the partially wetted plate of Fig. 3a: the force exerted by the fluid on the plate results from two contributions, as shown schematically in Fig. 15c. First, there is the vertical force component (per unit length) due to the vicinity of the contact line: ##\gamma(1 + \cos{\theta})## (cf. Fig. 12). Second, there are submerged surfaces of the plate where a localized curvature exists at the corners. This curvature induces a Laplace force [on the plate] which results into a net upward force ##\gamma_{LV}## per unit length of contact line which means the total force (per unit length of contact line) on the plate ##\gamma_{LV}\cos{\theta}##, in agreement with the thermodynamic result.
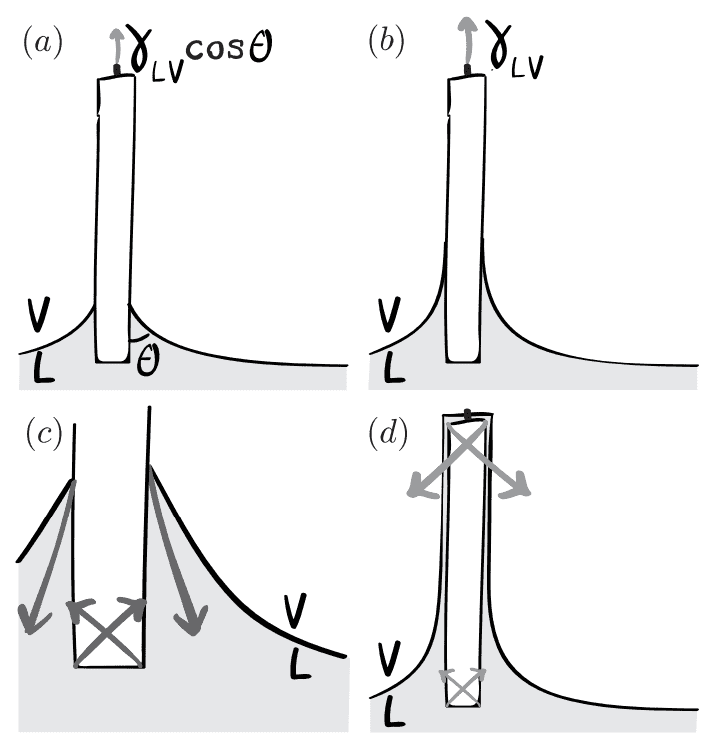
So there is an additional "Laplace force" caused by the curvature of the plate that has a vertical component per unit length of ##\gamma_{LV}##, which then yields the correct well known result that the surface tension causes an downward component of ##\gamma_{LV}(1+\cos{\theta}) - \gamma_{LV} = \gamma_{LV}\cos{\theta}##.
This is easier to imagine when the partially submerged plate has a lower face exposed to the liquid, but in the case of a liquid in e.g. a beaker, from where does the Laplace force arise?
Thanks!
Last edited by a moderator: