- #1
TK421
- 4
- 0
Homework Statement
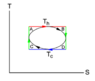
n moles of gas, that follow van der Waals equation are to be employed as the auxiliary system in a circular cycle(parameterized using T_h and T_c as shown on the TS diagram. Calculate the efficiency of the cycle.
Homework Equations


The Attempt at a Solution
Idea is simple. I just divide circle curve horizontally in two two curves. Using Q = TdS , by integration over the top curve i obtain Q_(in), by integrating over the bottom curve i obtain Q_(out). Then i easily get efficiency using equation above. The only problem is, that i don't know, how to calculate those integrals with only van der Waals equation of state initially given.