- #1
Milsomonk
- 96
- 17
Hi guys,
So I have a question in a piece of coursework which I seem to have done too simply, its worth 7 marks and I am thinking I've missed something somewhere so was hoping to run it by someone else.
1. Homework Statement
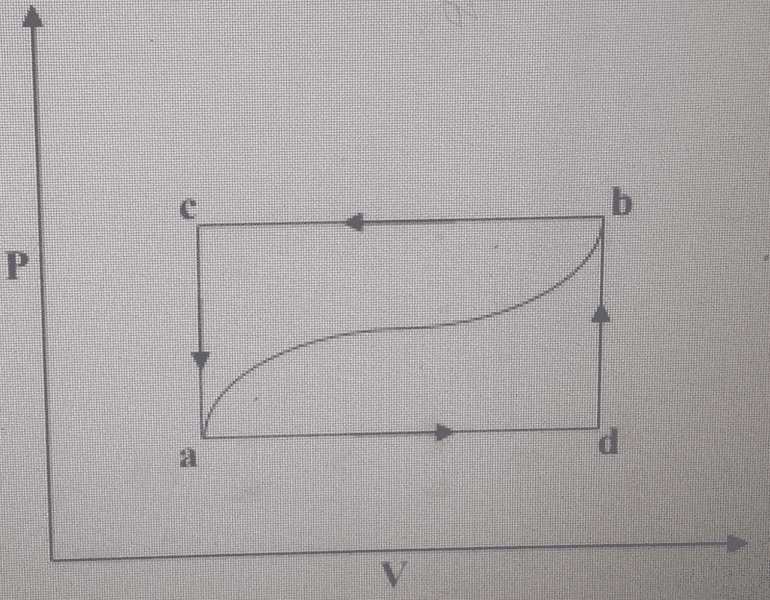
A system consists of gas contained in a cylinder fitted with a frictionless piston and
is taken from the state b to the state a along the path b d a shown in the
figure below. During this process 50 J of heat flow from the system and 25 J of
work is done on the system.
How much work is done when the system is taken along the path b c a if
40 J of heat are expelled by the system? Is it done on or by the system?
DQ=-DW
I have simply said that since 40J of heat flows from the system, 40J of work is done on the system. Due to the above equation. My only thought is that perhaps this equation doesn't hold for B to C to A since the path is not closed? If anyone can point me in the right direction I would be very grateful :)
Thanks
So I have a question in a piece of coursework which I seem to have done too simply, its worth 7 marks and I am thinking I've missed something somewhere so was hoping to run it by someone else.
1. Homework Statement
A system consists of gas contained in a cylinder fitted with a frictionless piston and
is taken from the state b to the state a along the path b d a shown in the
figure below. During this process 50 J of heat flow from the system and 25 J of
work is done on the system.
How much work is done when the system is taken along the path b c a if
40 J of heat are expelled by the system? Is it done on or by the system?
Homework Equations
DQ=-DW
The Attempt at a Solution
I have simply said that since 40J of heat flows from the system, 40J of work is done on the system. Due to the above equation. My only thought is that perhaps this equation doesn't hold for B to C to A since the path is not closed? If anyone can point me in the right direction I would be very grateful :)
Thanks
Last edited by a moderator: