- #1
krete77
- 17
- 0
Hey guys, this isn't a homework question or anything. I ended up lugging a lot of wood the other day and decided I would try to figure out on my own how to find out how many calories I burned.
I have a ton of data, but I'm just going to show you what I have done via this picture; hopefully you can see it.
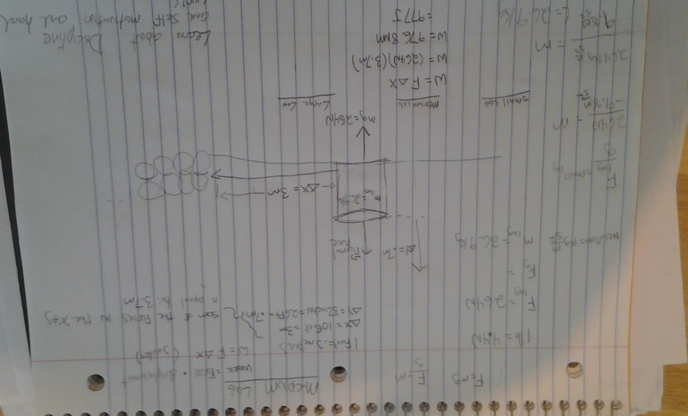
So the answer I came up with for the total amount of work done on a medium sized log was 977J. When I convert this to calories (977J*.239cal) I get 233cals. There's no way I burned 239 calories lifting one log .3m up, and walking it 3m away.
I used the 1 joule = 0.239005736 calories.
What am I doing wrong?
I have a ton of data, but I'm just going to show you what I have done via this picture; hopefully you can see it.
So the answer I came up with for the total amount of work done on a medium sized log was 977J. When I convert this to calories (977J*.239cal) I get 233cals. There's no way I burned 239 calories lifting one log .3m up, and walking it 3m away.
I used the 1 joule = 0.239005736 calories.
What am I doing wrong?