- #1
Alex Hughes
- 54
- 13
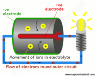
So I understand in a battery that an anode (such as zinc) and a cathode (such as carbon) are separated by an electrolyte. I also understand that the electrons want to flow into the cathode, but can't get to them, so as soon as a conductor connects the two terminals, current can flow. However, there are several questions I'm confused about and I was hoping somebody could help me. First, why zinc and carbon. Second, what is the purpose of the electrolyte? Why can't charges just flow through the battery to the other electrode, does the electrolyte stop that from happening? Lastly, what actually makes the electrons in the anode want to move to the cathode. Everybody just says that they want to but not why. Sorry for rambling I'm just frustrated that nobody can give me a clear answer.