- #1
Qube
Gold Member
- 468
- 1
Hybridization of carbon
Pretty sure my professor is wrong once again.
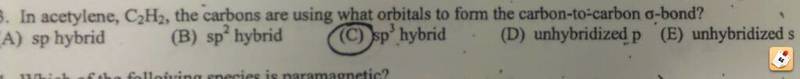
SO2 should have two double bonds which gives the sulfur a minimal formal charge and two signs bonds. The two sigma bonds imply sp hybridization, not the blatantly wrong circled answer.
Pretty sure my professor is wrong once again.
SO2 should have two double bonds which gives the sulfur a minimal formal charge and two signs bonds. The two sigma bonds imply sp hybridization, not the blatantly wrong circled answer.
Last edited: