- #1
Voltux
- 30
- 3
TL;DR Summary: I measured the mass of CO2. Can someone verify my calculations?
I took a soda bottle and filled it with Carbon Dioxide then weighted its mass as I changed pressures. My figures get very close (5-10%) within the calculated values for psig but not psi-absolute. Can someone confirm my results are correct? I think of this as confirmation bias but in a way I expected the values to be closer to the psia instead of psig.
Procedure
An empty "2L" bottle was filled with water to verify its actual volume. Approximately 2054g (mL) was measured on an 0.1g resolution electronic Ohaus balance.
From this the bottle was then emptied, weighted again after drying, then squeezed out of air followed by being filled with CO2 through a one-way valve. The bottle was then incrementally weighed followed by adjusting the pressure on the regulator and repeating the experiment for the values noted.
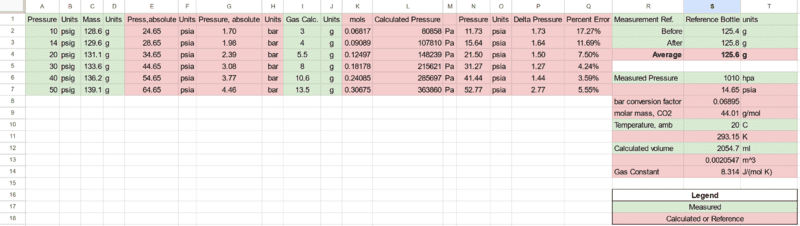
After the experiment values were placed in a spreadsheet, and calculated to adjust units along with measurements for ambient pressure and temperature were taken from similar electronic instruments.
The experiment suggests that the relationship between pressure and mass are linear in nature over the range explored in this experiment. There is expected to be some error from the reference pressure point since it was obtained by an analog gas regulator gauge.
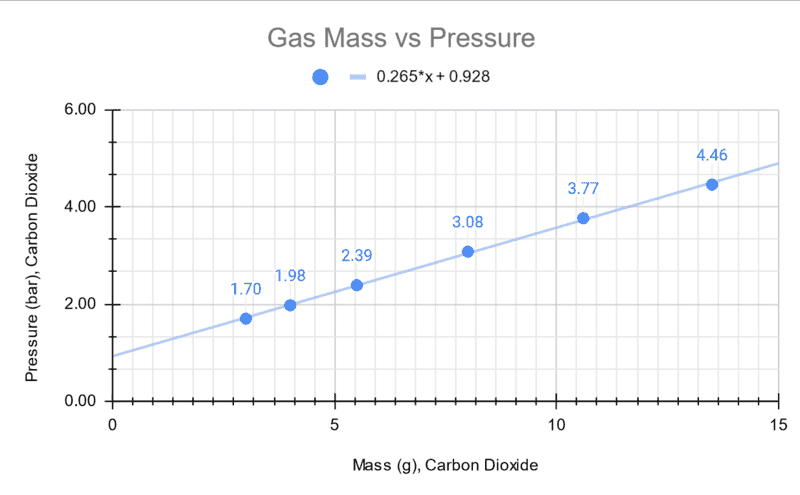
I took a soda bottle and filled it with Carbon Dioxide then weighted its mass as I changed pressures. My figures get very close (5-10%) within the calculated values for psig but not psi-absolute. Can someone confirm my results are correct? I think of this as confirmation bias but in a way I expected the values to be closer to the psia instead of psig.
Procedure
An empty "2L" bottle was filled with water to verify its actual volume. Approximately 2054g (mL) was measured on an 0.1g resolution electronic Ohaus balance.
From this the bottle was then emptied, weighted again after drying, then squeezed out of air followed by being filled with CO2 through a one-way valve. The bottle was then incrementally weighed followed by adjusting the pressure on the regulator and repeating the experiment for the values noted.
After the experiment values were placed in a spreadsheet, and calculated to adjust units along with measurements for ambient pressure and temperature were taken from similar electronic instruments.
The experiment suggests that the relationship between pressure and mass are linear in nature over the range explored in this experiment. There is expected to be some error from the reference pressure point since it was obtained by an analog gas regulator gauge.
Attachments
Last edited: