- #1
MatinSAR
- 528
- 174
- Homework Statement
- How do I find the volume-pressure equation for an expanding ideal gas for the figure below?
- Relevant Equations
- pV=nRT
There is no atmosphere pressure.
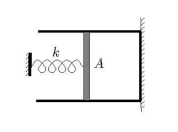
My work :
pA=k(x-x0) => pA=(k/A)(V-V0)
But this should be false beccause I want to use W=∫PdV to find work done by the gas but my final anwer is wrong ...
Please guide me where my mistake is if you have enough time. Thanks.
My work :
pA=k(x-x0) => pA=(k/A)(V-V0)
But this should be false beccause I want to use W=∫PdV to find work done by the gas but my final anwer is wrong ...
Please guide me where my mistake is if you have enough time. Thanks.