- #1
Quantum Velocity
- 73
- 6
Hey guy! I've just started learn physics and i ran into a problem.
Can you guy let me know what is d and p mean in this
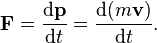
Can you guy let me know what is d and p mean in this
Force is mass times acceleration, and acceleration is the time derivative (rate of change) of velocity. You are going to need calculus to understand this.Quantum Velocity said:But what dẻivative got to do with this equation
The d and p symbols in physics refer to the orbital quantum numbers that define the shape and energy of an electron's orbit around an atom's nucleus.
The main difference between d and p orbitals is their shape. The p orbitals are dumbbell-shaped and oriented along the x, y, and z axes, while the d orbitals have more complex shapes and are oriented along the x, y, z, xy, xz, yz, x2-y2, and z2 axes.
The d and p orbitals play a crucial role in determining an atom's chemical and physical properties. For instance, the number of electrons in the d orbitals can affect an atom's magnetic properties, while the orientation of the p orbitals can impact its reactivity with other atoms.
The number after the d or p symbol represents the energy level or shell of the orbital. For example, the 3d orbital is in the third energy level, while the 2p orbital is in the second energy level.
The d and p orbitals are associated with the transition metals and the main group elements, respectively, in the periodic table. The elements in these groups have different numbers of electrons in their d and p orbitals, which can explain their unique properties and chemical behaviors.