- #1
orgmann
- 4
- 0
Among the following compounds I think D compound has more rate of solvolysis in 50% aq.ethanol at 45°C than compound C .
Due to more resonance.
But the compound C has more rate . Why?
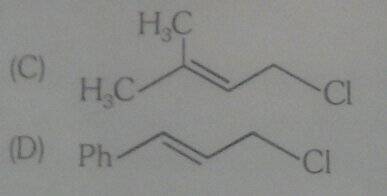
Due to more resonance.
But the compound C has more rate . Why?