- #1
pangru
- 15
- 2
According to Einstein (or Debay) model of solids, heat capacity drops exponentially at low temperatures:
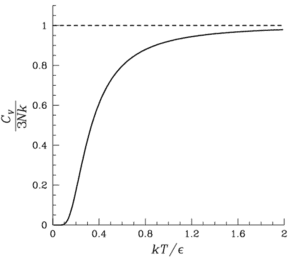
question is >> why it changes so dramatically at low temperature
that is physical explanation of this?
question is >> why it changes so dramatically at low temperature
that is physical explanation of this?