- #1
persia77
- 25
- 0
if in a solid there arent no states with same energy then why energy band vs location diagram is flat?
but pauli exclusion principle states that there are not same states in a solidNaOH said:The energy states are spread throughout the whole solid. So each horizontal line represents the same state.
persia77 said:but pauli exclusion principle states that there are not same states in a solid
if you are correct then two electron can occupy same energy state but pauli exclusion principle states that they cannotNaOH said:The same energy state is extended throughout the solid, in other words, the electron wave is spread throughout the whole solid.
persia77 said:if you are correct then two electron can occupy same energy state but pauli exclusion principle states that they cannot
is it correct?
if you are correct then a electron doesn't have position in solid and electron move through the solid but in valance band electron is not free and don't moveNaOH said:I did not mean to imply that two electrons occupy the same state. I am saying that the electron is spread throughout the whole solid. However, as we move along the crystal and we are still talking about the same electron (and thus state), the line is diagrammatically shown to be horizontal. The electrons still occupy states as allowed by pauli's exclusion principle.
persia77 said:if you are correct then a electron doesn't have position in solid and electron move through the solid but in valance band electron is not free and don't move
persia77 said:if in a solid there arent no states with same energy then why energy band vs location diagram is flat?
i say about valance(bonding) electron not free electronNaOH said:To understand this you need another piece of knowledge which is not found in your typical band diagram -- that of (crystal) momentum.
Associated with each energy state is a certain (crystal) momentum. This momentum is what allows the net movement of electrons, which we measure as current. When we apply an electric field, we can describe it as shifting all the electron states with some momentum. In metals, in general, this happens without any huge problems because the shifting is done by occupying the conduction band states.
In insulators, things behave slightly differently. Now, when we apply the electric field, we are not able to shift the electron states! Why? Because of the band gap -- there are no states to be shifted to.
In semiconductors, we get to access some of these states, but usually it is more than we need.
i say about real space not k spaceZapperZ said:This is the full band structure of copper:
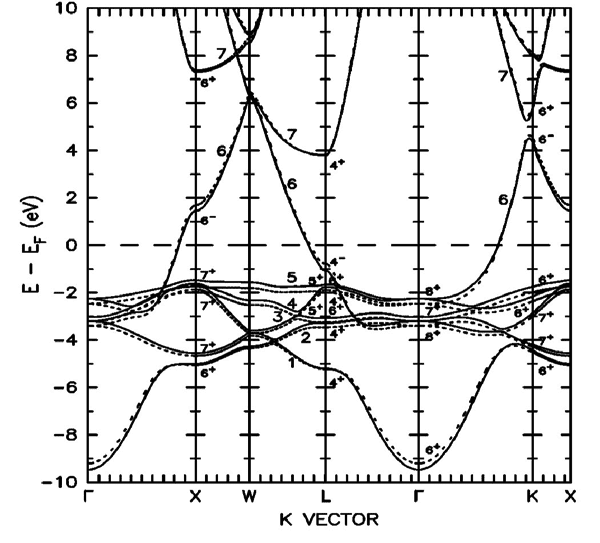
You'll notice that it isn't "flat", especially the conduction band.
As NaOH is trying to tell you, your band diagram is a "cartoon" picture simply to indicate where the available states are. It is really a reduced picture where the momentum k has been integrated out, leaving only the available energy states. This means that the electrons in the bands are characterized by not just energy, but also momentum (E, k). So while they may occupy the same energy, there is degeneracy in the form of differing momentum, resulting in a band dispersion. This does not result in an overall "flat" curves.
Zz.
persia77 said:i say about real space not k space
please show real space diagram
persia77 said:i say about valance(bonding) electron not free electron
valance electron is fixed not moving
you say there are same energy state in solid ,it is contradicted with pauli exclusion principle
persia77 said:i say about valance(bonding) electron not free electron
valance electron is fixed not moving
you say there are same energy state in solid ,it is contradicted with pauli exclusion principle
if they move then what is diffence between them and free electron?NaOH said:When the valance band is full, the electrons are still moving all over, but there is no net movement because electrons that travel left are bal.
The difference between them and free electron is effective mass. In solids, you must understand that bulk properties are usually not the result of each single electron minding their own business but rather the effect of the collection of all their properties.persia77 said:if they move then what is diffence between them and free electron?
why we can define effective mass only for free electron?
please show me a textbook that say your statementNaOH said:it moves throughout the whole crystal.
Introduction of Solid State Physics, charles kittel.persia77 said:please show me a textbook that say your statement
thanksNaOH said:Introduction of Solid State Physics, charles kittel.
Look under bloch functions
I can't help you any further. What I can say is that the book mentions the form of wavefunction for traveling waves, for another the Bloch function is a (travelling) plane wave.persia77 said:thanks
but its not mentioned there
The energy band diagram is flat in certain materials because these materials have a completely filled valence band and an empty conduction band. This means that there is a large energy gap between the two bands, and no electrons can move freely between them. As a result, the energy band diagram appears flat.
The shape of the energy band diagram is determined by the electronic structure of the material. Specifically, it is influenced by the number of valence electrons, the atomic structure, and the bonding between atoms. These factors determine the energy levels of the electrons and the size of the band gap.
Yes, the energy band diagram can be modified by introducing impurities or defects into the material. This can create additional energy levels within the band gap, allowing electrons to move between the valence and conduction bands. This process is known as doping and is commonly used in semiconductor materials to control the flow of electrons.
The flat energy band diagram means that the material is an insulator, as there are no available energy levels for electrons to move through. This results in low electrical conductivity. However, as previously mentioned, doping can modify the energy band diagram and increase the conductivity of a material.
No, the energy band diagram varies for different materials based on their electronic structure. For example, metals have overlapping valence and conduction bands, resulting in high conductivity. Insulators, on the other hand, have a large energy gap between the two bands, making them poor conductors. Therefore, the energy band diagram is not the same for all materials.