- #1
ChiralSuperfields
- 1,216
- 132
- Homework Statement
- Please see below
- Relevant Equations
- Please see below
For this problem,
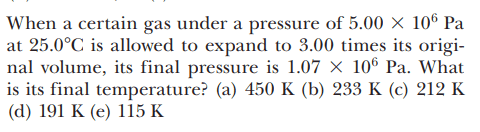
The solution is,
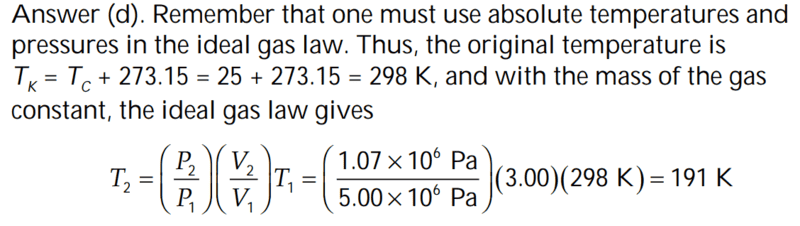
However, why must we use absolute temperature for the ideal gas law (i.e why can we not use Celsius for T)
Many thanks!
The solution is,
However, why must we use absolute temperature for the ideal gas law (i.e why can we not use Celsius for T)
Many thanks!