You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
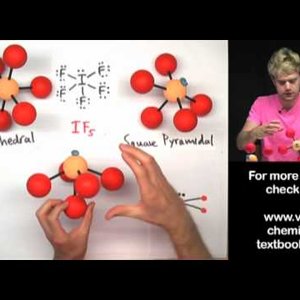
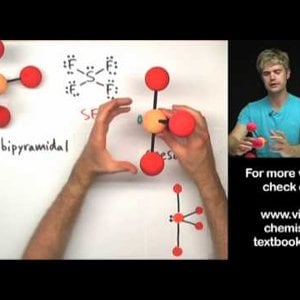
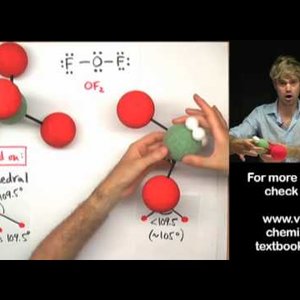
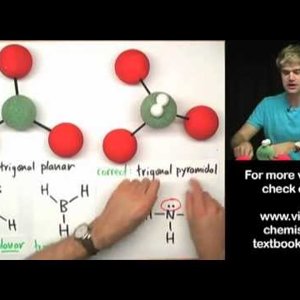
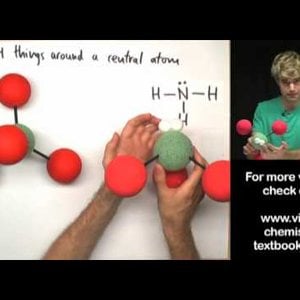
Don't make these common mistakes with VSEPR! This video talks about how to determine the shape or geometry of a molecule using the VSEPR rules, for valence shell electron pair repulsion. Unshared electron pairs (also known as lone pairs) are very important for determining geometry.