You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Acids and bases: Definition and 49 Discussions
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.
Their importance becomes apparent in analyzing acid–base reactions for gaseous or liquid species, or when acid or base character may be somewhat less apparent. The first of these concepts was provided by the French chemist Antoine Lavoisier, around 1776.
It is important to think of the acid-base reaction models as theories that complement each other. For example, the current Lewis model has the broadest definition of what an acid and base are, with the Brønsted-Lowry theory being a subset of what acids and bases are, and the Arrhenius theory being the most restrictive.
View More On Wikipedia.org
Their importance becomes apparent in analyzing acid–base reactions for gaseous or liquid species, or when acid or base character may be somewhat less apparent. The first of these concepts was provided by the French chemist Antoine Lavoisier, around 1776.
It is important to think of the acid-base reaction models as theories that complement each other. For example, the current Lewis model has the broadest definition of what an acid and base are, with the Brønsted-Lowry theory being a subset of what acids and bases are, and the Arrhenius theory being the most restrictive.
View More On Wikipedia.org
-

Chemistry Bronsted-Lowery Help: Understanding HCOOH + CN- and H2O + HCO3- Reactions
1) HCOOH + CN- ---> HCOOH- + HCN 2) H2O + HCO3- <===> H3O+ + CO3-2- stuidvkook
- Thread
-
- Tags
- Acids and bases
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-

Liming Lakes: A Solution for Acidification?
I calculated it and found that is approximately 610Kg needed of CaCO3 to bring the lake pH to a neutral level. Is my calculation right 1st :)? Then if this is right, 610kg of CaCO3 is not that much in a matter of cost or weight to help the bio-life of the lake. I'm wondering if we are applying...- StarChem
- Thread
-
- Tags
- acids and bases chemistry ph
- Replies: 4
- Forum: Chemistry
-

Approximations in Chemical Equilibrium (add a weak acid HA into pure water)
Suppose we add a weak acid HA into pure water, so that upon addition its initial concentration is c. The following equilibria should establish in the system. $$\text{HA}+\text{H}_2\text{O}\rightleftharpoons\text{H}_3\text{O}^++\text{A}^-$$...- SilverSoldier
- Thread
- Replies: 3
- Forum: Chemistry
-
C
Chemistry Factors that determine acid strength
- chemJan2021
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Calculations involving acids and bases
Summary:: finding ml of two solutions by the final pH i have a NaOAc 0.1M and HOAc 0.1M , together the volume of the solutions is 20ml and the pH is 4. I need to find the volume of each solution. I've tried to solve it for hours with no successes. i found the H+ concentration (-log(h)=4 ), it...- sergey_le
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
R
I am sure I am doing this Acid/Base problem wrong
What is the pH of a solution made from 34.6 ml if 3.45*10^-4 M of H2SO4 added to 41.7 ml of 4.56*10^-4 M of Al(OH)3? I know how to find H+ and OH- and pH I also know how to use x mol / L, but I cannot get them to click together. Everyone keeps telling me to use RICE but my professor never...- Renee Crosby
- Thread
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-

How can I distinguish between Lewis acids and bases?
How do I know which species is a Lewis acid and which is a Lewis base?- sidt36
- Thread
-
- Tags
- Acids Acids and bases Concepts
- Replies: 2
- Forum: Chemistry
-
T
Pouring acids and bases over sinks
Homework Statement Which of the following would you not do in a laboratory setting? I. Pour acids and bases over a sink II. Wear goggles III. Heat a stoppered test tube (A) I only (B) II only (C) III only (D) I and III only (E) I, II, and III Homework Equations - The Attempt at a...- TT0
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
E
Acids & Bases: Aspirin pKa, pH in Stomach & Intestine
Homework Statement The pKa of acetylsalicylic acid (aspirin) is 3.5. The pH of gastric juice in the stomach is about 2-3 and the pH in the small intestine is about 8. Aspirin will be Answer is ionised in the small intestine and almost unionised in the stomach. Homework EquationsThe Attempt at...- erisedk
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-

Why equilibrium favours weak acid or weak base
Homework Statement Which side does the equilibrium favour? Homework Equations Equilibrium favours weak acid or weak base (I don't know why :frown:) The Attempt at a Solution Using the given pka values, since the reactant is the weaker acid, reactants are favoured. (Backward reaction is...- Titan97
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
N
When to Use Kw & Ka for Acid Dissociation Calculations
So I've learned that in strong acids that dissociate completely, the concentration of H+ is the same as the concentration of the initial solution. So 1M of a strong acid will create 1M of H+, meaning the pH is 0. I've also learned that in weak acids, the whole thing doesn't dissociate. I have...- Nishantkumar19
- Thread
- Replies: 6
- Forum: Chemistry
-

A question about the special case of calcium hydroxide
Hi! I am having trouble understanding how a substance only slightly soluble in water is considered as a strong base. Isn't the definition of a base a substance that will increase the amount of OH- in a solution? In that case, shouldn't calcium hydroxide be considered a weak base because of its...- Dong Aleta
- Thread
- Replies: 5
- Forum: Chemistry
-

A question regarding the definition of acids and bases
Hi! I just read in an inorganic chemistry book (by Whitten, et al.) that acids are defined as substances that produce H+ ions in dilute aqueous solutions, and bases are those that produce OH-. To me, this definition implies that a substance that has yet to produce an H+ or an OH- can already be...- Dong Aleta
- Thread
- Replies: 3
- Forum: Chemistry
-
G
What is the common property shared by all acids that leads to their definition?
I've written the following brief history on how the definition of acids and bases change over time. Lavoisier came up with his definition circa 1776 in that 'p is acidic iff p contains much oxygen' and that held sway for 30 years[1] until it was falsified by Davy in 1810[2] who demonstrated...- gamow99
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 4
- Forum: Chemistry
-
V
Selective precipitation, purity and yield of precipitate
Homework Statement An aqueous effluent contains 12 M Cd2+ and 10 M Mg2+ as solution of nitrates. Current practice is use NaOH to selectively precipitate the metals. (a) Is it feasible to get 0 Cd impurities in Mg ppt by using NaF instead of NaOH (back up your answer with adequate calculations)...- vickyvoo2
- Thread
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
J
Procedure of preparation of HCl
Homework Statement Knowing that HCl is strong acid we want to prepare HCl solution of pH=4.2.starting from a solution of pH=2.9.Explain briefly the procedure that should be followed to do this operation. Homework Equations pH=-log(H3O+) 3. The Attempt at a Solution [/B] I calculated: At...- Jeanclaud
- Thread
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
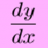
Need help with (Acids and Bases) high school exam
< Mentor Note -- thread moved to HH from the technical PF forums, so no HH Template is shown > Hello! So our teacher puts some really hard questions in acids and bases exams, and I would like someone to help me find similar questions and know how to solve these questions.. So the first...- iwantcalculus
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
C
Calculating pH and pOH for 0.048 M Benzoic Acid Solution
Homework Statement what is the H, Oh , ph and poh for a 0.048 mol/l solution of benzoic acid? C6H5COOH Homework Equations Ph +poh=14 Ph=-log(h30+) H30=10^-ph The Attempt at a Solution I thought maybe u need to separate benzoic acid into its ions and then use the molar ratio to...- Coco12
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
2
Calculating H3O+ Concentration for Acetic Acid Solution | Ka = 1.8 x 10-5
Homework Statement What is the H3O+ concentration of a 0.100 M acetic acid solution (Ka = 1.8 x 10-5)? a. 1.8 x 10-5 b. 1.8 x 10-4 c. 1.3 x 10-2 d. 1.3 x 10-3 e. 0.9 x 10-3 Homework Equations The Attempt at a Solution so the question is supposed to be D. but I thought that...- 2200andbeyond
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
Acid-Base Reactions: Is it Always a One-to-One Relationship?
Lets say a certain chemical reacts with an acid. Does that make that chemical a base always and vice versa? Like in S + NaOH → Na2S + Na2S2O3, Sulfur reacting with a base would it make S an acid? Or do we call it a base or an acid, reacting with the opposite if it's producing water as a...- Knightycloud
- Thread
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
J
Acids and Bases and Their Reactions
Homework Statement Oxalic acid, H2C2O4, which is found in certain pants, can provide two hydronium ions in water. Write balanced equations to show how oxalic acid can supply one and then a second H3O+ ion. Homework Equations There's not really an equation, and if there is please don't make...- jbmiller
- Thread
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
M
Separation using acids and bases
I am doing a lab this week where the objective is to use acids and bases and a microscale extraction to separate three solids in a mixture. The mixture in question is a mixture of naphthalene, benzoic acid, and ethyl 4-aminobenzoate. It will then be put through a series of solvents and...- member 392791
- Thread
- Replies: 1
- Forum: Chemistry
-
K
Acids and Bases calculation problems
Homework Statement Data for Question B: (initial)[CH3COOH] = 0.500 mol dm–3 and) eqm [CH3COOH] = 0.200 mol dm–3; (initial)[CH3COO–] = 0.300 mol dm–3 and) eqm [CH3COO–] = 0.300 mol dm–3; The Attempt at a Solution Question A) " FInd the pH of a mixture of 50.0 cm3 of 0.100 mol...- kenshi64
- Thread
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
R
Acids and Bases. How to tell if a compound is an acid or base or neautral?
I've been given a question on how to tell whether a compound is an acid or base or neautral in an aquesous solution. Na2CO3 Please help.- Raishah
- Thread
-
- Tags
- Acid Acids Acids and bases Base Bases
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
D
Why can the mixing of acids and bases be dangerous
I tried googling this topic and basically all I could find was sites saying they neutralise each other - I know that. But why is it that when you mix say a drop of pH 14 solution with a lot of pH 1 solution that they react rather violently and the acid sprays everywhere? If someone could maybe...- Da Apprentice
- Thread
-
- Tags
- Acids Acids and bases Bases Mixing
- Replies: 5
- Forum: Chemistry
-
P
Why Do My Amphoteric Equations Keep Going Wrong?
Homework Statement I'm having problems understanding amphoteric acids and bases. I know amphoteric means that it can act as an acid or base, but when the question asks me to write the equation to support this statement, I always get it wrong :confused: Homework Equations HSO4^-1 is an...- paperdoll
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
U
Understanding Lewis Acids & Bases: HCl, H2SO4, H3PO4, CO2, HCN
hi all! I am not able to understand what's a Lewis acid and a Lewis base. tell me if these are Lewis acids or Lewis bases and tell me the reason. HCl, H2SO4, H3PO4, CO2, HCN. my approach: first 3 I'm not able to decide. 4th one I thought to be Lewis base as 2lone pairs of electrons left in...- utkarsh009
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
B
Simple acids and bases question
Sorry guys, I can't seem to grasp the concept: It deals with acids and bases. When the acid donates the proton, to the base, how do we know how many is transferred and how many the base can accept? This is where I am stumped. Homework Statement What ions are formed when nitric...- baylin
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
P
How do I classify acids and bases in chemistry?
Can anyone explain to me how to determine whether a compound is a strong acid, weak acid, strong base, weak base, or amphoteric? Thanks!- Prone17
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 5
- Forum: Chemistry
-
L
Neutralization reaction: NaCl + H2O
This is a subject I am unfamiliar with, and was just wondering where I should start in order to learn about them and how they affect different elements and form other substances step-by-step. From what I know, an Acid donated a Hydrogen Ion, and a Base takes a Hydrogen Ion, and an Acid has to...- LogicalAcid
- Thread
- Replies: 8
- Forum: Chemistry
-
L
Weak acids and bases buffers
Homework Statement I'm a little confused, let's say i do a titration curve for a weak acid, at some point there's a buffering range near the pKa. Like i know that if we add a strong base like NaOH to acetic acid in a water solvent, then the OH- will combine with the H+ (hydronium ion) which...- lha08
- Thread
-
- Tags
- Acids Acids and bases Bases Buffers Weak
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
W
Mixtures of Acids and Bases: What Type of Solution is Produced?
Homework Statement You prepared a reaction table for 10.0 mL of 0.1 M NH4Cl with 5.0 mL of 0.1 M NaOH. Please choose all of the following that describe the solution that was produced. The choices are: 1. a solution containing only a strong base 2. a solution containing a weak acid and a...- w3390
- Thread
- Replies: 18
- Forum: Biology and Chemistry Homework Help
-
L
Differentiating Acids and Bases Based on Chemical Formulas
Homework Statement does anyone know how i can differentiate a strong acid from a weak acid and likewise for a strong base and a weak base based on its chemical formula? Homework Equations The Attempt at a Solution- lha08
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
S
Chemistry AP - Acids and bases
I was looking for information in regard to Acids and Bases. I'm having a awful time with conceptual questions concerning this chapter in class, and I'm getting pretty frustrated. Any practice questions / supplemental in depth tutorials would be nice. Thanks!- Skynt
- Thread
-
- Tags
- Acids Acids and bases Ap Bases Chemistry
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
D
Experimenting with Acids & Bases: DeltaH, Dissociation & Bond Strength
I did an experiment with a) strong acid (HCl) and strong base (NaOH) b) weak acid (ethanoic) and strong base (NaOH) so, I am looking at the heat of energy for both the reactions, and so i found that reaction a had a greater heat of energy was that of the reaction b) weak acid and...- ditto_299
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
D
Bronsted - Lowry Theory Of Acids and Bases
Homework Statement Some of the books I have state that the equation of... HCl + NH3 --> NH4+ + Cl- While others state that the above equation should be written as... HCl + NH3 --> NH4Cl Which one is correct? I think the answer should be the second one since the two compounds...- DarylMBCP
- Thread
-
- Tags
- Acids Acids and bases Bases Theory
- Replies: 25
- Forum: Biology and Chemistry Homework Help
-
C
Determining the Most Effective Buffer Solution for pH Stability
Hi there I was wondering if anyone could help me with this buffers question ... For which of these liquids is the pH likely to change least if a splash of concentrated sulphuric acid is added? Justify your answer: a) 0.1M hydrochloric acid solution b) 0.08M ethanoic acid/0.02M potassium...- cookie13
- Thread
-
- Tags
- Acids Acids and bases Bases Buffers
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
P
I have my acids and bases test tomorrow, and i with a few types of problems
im looking at some review problems my teacher gave me today, and i can pretty much do most of them. however, there is this one i really have no idea what to do. Its a 2 part problem. 1a.What is the pH of a 1000 ml of a 0.1 M acetic acid solution, given pKa = 4.8? 1b. what is the pH of...- pakmingki
- Thread
-
- Tags
- Acids Acids and bases Bases Test
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
L
Understanding Bronsted Acids and Bases: Classification and Examples
Anyone clever with bronsted acids and bases? I'm pretty new in chemistry, but I'm trying to learn. Right now I'm suppose to classify some species as either bronsted acids or bases, or even both. For example 1. Water 2. OH- 3. NH4+ 4. HCN 5. HBr 6. NH3 Hope someone can help...- Lookielook
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
B
Acids and bases chemistry problems
I am a little stuck on this question : Solution A contains n moles of a weak acid HX. The addition of some sodium hydroxide to A neutralises one third of the HX present to produce Solution B. In terms of the amount, n how many moles of HX are present in Solution B? I think there are one...- busted
- Thread
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
D
Solving Organic Chemistry: Acids & Bases Problems
I have problem in complete understanding these concepts especialy in organic chemistry. Inorganic was simple HaON, HCl and similar, but now in organic chemistry Acids and Bases concept and fast determining of those is killing me :(. Is there a place on internet with good tutorial covering...- Dr. Nick
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 2
- Forum: Chemistry
-
A
Finding pH Values for Acids and Bases Without Experiments
I have to place two acids and two bases from a previous assignment on a pH scale using the information I acquired during that particular experiment. The problem is, I couldn't get all the materials I needed so I didn't get to do the assignment. I've been able to figure out what vinegar's pH is...- AngelShare
- Thread
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
D
Is My Acid-Base Mixture Actually Neutral?
In my chmeistry lab i use a pipet 5.0 ml of HCl and add it to a beaker. To this beaker I also added 5.0 ml NaOH. I stir the mixture and when a red litmus it turn to blue and when i put a blue litmus it stay blue. So that means its a base, but since its a mixture of an acid and a base shouldn't...- david2120
- Thread
-
- Tags
- Acids Acids and bases Bases Chem Lab
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
D
What Determines the Strength of a Conjugate Acid-Base Pair?
Uggh finally did all the chem problems and studied for the upcoming final but there are 2 questions left unanswered. MAybe someone can help me out with this: If given a lists of acids, how would one know which 1 is the weakest conjugate base? For example, HF, HNO_2, H_2CO_3, H_3BO_3, HCl... -
J
Calculate pH & Concentration of Acids & Bases in Solution
Could You Help Me Out With This Question Please: 25.0 cm3 of a solution of 0.1M ethanoic acid is titrated with 0.1M sodium hydroxide.When sufficient alkali has been added to neutralise half of the acid,calculate a)the concentration of ethanoic acid and ethanoate ions b)the pH of the...- Jack16
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 4
- Forum: Chemistry
-
J
Solving Acids & Bases Questions - pH, Ionisation Constant
Hi People , I had a problem solving this question,could you please help me:D The question: a)The ionisation constant for propanoic acid is 1.26x10^-5 mol dm^-3.Calculate pH of a 1.0M solution b)What is the hydroxide ion concentration of this solution? c)Pocroc acid has an ionisation...- Jack16
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 13
- Forum: Chemistry
-
J
pH of Pure Water at 100°C | Acids & Bases
Could You Help Me Out Please... The Value of Kw ,the ionic product of water,increases with temperature.Will the pH of pure water be greater or less than 7 at 100*C?- Jack16
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 2
- Forum: Chemistry
-
J
Explaining Difference in pH Values of Hydrocloric & Ethanoic Acids
Hello People, I am a new member and i really think this forum is very helpful... I got a question... The pH values of 0.100moldm-3 solutions of hydrocloric acid and ethanoic acid are 1 and aproximately 3 respectively. Explain the difference. Thanks already...- Jack16
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 3
- Forum: Chemistry
-
E
Explaining Acids and Bases to a Six-Year-Old
What are some good ways to explain acids and bases to a bright six year old?- Entropia
- Thread
-
- Tags
- Acids Acids and bases Bases
- Replies: 2
- Forum: Chemistry