Kerrigoth
- 14
- 1
The book I'm reading is discussing the physics of semiconductors. I'm having a hard time understanding a passage in section introducing n-type semiconductors.
(Phosphorus is used as the impurity)
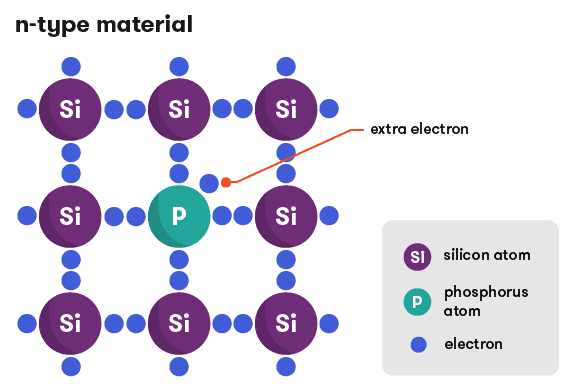
The book says:
I've been under the impression holes are regarded as positive charge because when an electron is absent the atom becomes ionized. This ionization is why the hole is considered positive. How could the atom be ionized without a hole present?
So why would the author say a hole is not created after an electron becomes free? This missing electron's vacancy could be filled by other extra electrons contributed by other phosphorus atoms in the doped material.
Thanks for your time.
(Phosphorus is used as the impurity)
The book says:
"At normal operating temperatures, this extra electron breaks its bond with the impurity atom and becomes a free electron. However, a hole is not created by the impurity atom--the positive charge that balances the free electron is locked in the ionic core"
I've been under the impression holes are regarded as positive charge because when an electron is absent the atom becomes ionized. This ionization is why the hole is considered positive. How could the atom be ionized without a hole present?
So why would the author say a hole is not created after an electron becomes free? This missing electron's vacancy could be filled by other extra electrons contributed by other phosphorus atoms in the doped material.
Thanks for your time.