Thanks Davenn for your responses.
They say (I don't know, I've never actually researched it. I just read it in the net) that the composting process is the reverse of photosynthesis. That is
C
6H
12O
6 + 6O
2 -> 6CO
2 + 6H
2O + heat.
Someone said in PF forum, that the reverse of photosynthesis is true, but the process is not that simple, it involved nitrogen and some other element.
Okay...
So the composting expelled CO
2 and water,
But my composter bin, I think, is for
anaerobic composting.
So, it does not expleled CO
2 and water. Instead it expelled CH
4 and H
2S.
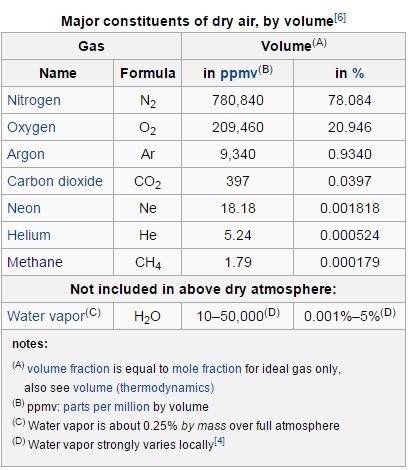
But before I go further, let me give you the atmosphere facts first.
By volume the atmosphere contains:
N
2 78.08%
O
2 20.94%
Ar 0.93%
CO
2 0.0397%
the other Ne, Helium, Methane are too small fraction.
List of molecular weight compared to H -> 1.008. I divide those number by Hydrogen weight, so it will be an easier conversion.
Nitrogen: 27.8
Oxygen: 31.75
Argon: 39.63
CO
2: 43.66
Multiply those numbers by volume divided by total volume divided by Hydrogen weight, I have the weight of the Earth atmosphere is
28.74 times of the weight of Hydrogen.
The weight of CH
4 compared with H is 15.91
The weight of Hydrogen Sulfida compared with H is 33.82
Okay, now back to your question:
davenn said:
Stephanus said:
Second, I think the gas pressure inside the composter (because there are some chemical reaction in it, bacteria digesting) is higher than the atmosphere, so gas will be expelled from both lower and upper hole.
That may have an effect, but I was thinking of something more significant ... have another think about processes occurring in the compost pile
I think I stick to my earlier answer. The gas in the composting will be expelled
IF the pressure in the composter bin is higher than the atmospheric pressure, not matter what gas inside. I've already calculated the density of the gas and atmosphere. But on second thought I think that doesn't matter. Only the pressure in the composter bin matters.
If I were a detective, I would have lost this case. I'm sorry, I'm lost here. What do you think why the oxygen is not likely enters the composter bin from above? Is it
gas pressure?
Thanks
Steven