edguy99
Gold Member
- 449
- 28
The empty shell provokes some interesting thought when considering the orbit of an electron. Assume there is no proton/electron coulomb force if the electron is inside the proton shell.
Some simulations. First is an electron orbiting a proton with enough energy that it gets a reasonable orbit. The second simulation shows a lower energy electron completely trapped within the proton. The third simulation shows the kind of orbits you get with more objects (two electons and one proton).
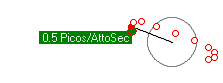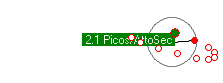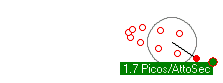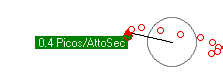 [PLAIN]http://upload.wikimedia.org/wikipedia/commons/7/7c/Protonelectrontrapped.gif[ATTACH=full]196589[/ATTACH][/URL]
[PLAIN]http://upload.wikimedia.org/wikipedia/commons/7/7c/Protonelectrontrapped.gif[ATTACH=full]196589[/ATTACH][/URL]
Some simulations. First is an electron orbiting a proton with enough energy that it gets a reasonable orbit. The second simulation shows a lower energy electron completely trapped within the proton. The third simulation shows the kind of orbits you get with more objects (two electons and one proton).
Attachments
Last edited by a moderator: