SUMMARY
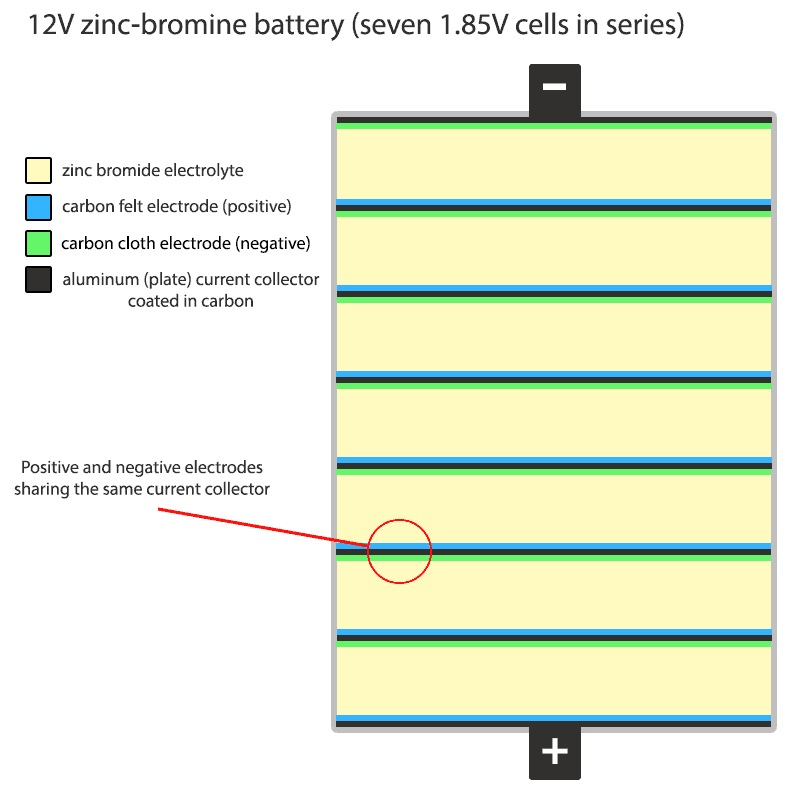
Positive and negative electrodes in series can indeed share the same aluminum current collector, which is coated in carbon. This configuration allows for the construction of a 12V battery without the need for individual current collectors for each cell. The design is membrane-free and effectively manages zinc dendrite formation by utilizing a corrosive bromine solution that dissolves dendrites back into the electrolyte. The battery case is made of HDPE plastic, ensuring durability and safety.
PREREQUISITES
- Understanding of electrochemical cells and battery design
- Knowledge of current collector materials, specifically aluminum and carbon coatings
- Familiarity with zinc dendrite formation and its implications in battery performance
- Basic principles of series battery configurations
NEXT STEPS
- Research the effects of zinc dendrites on battery efficiency and lifespan
- Explore advanced materials for current collectors in battery applications
- Investigate the design and performance of membrane-free battery systems
- Learn about the properties and applications of HDPE in battery casing
USEFUL FOR
Battery engineers, materials scientists, and anyone involved in the design and optimization of electrochemical cells and battery systems.