- #1
ifihadsomebacon
- 4
- 0
Going from:
 E = q + w
E = q + w
To:
 H =
H =
 E +
E +
 (PV)
(PV)
I'm confused as to why you add the product of the pressure and volume of the system to the internal energy to get enthalpy. Is it just because "that's what enthalpy is defined as"? I think I understand that when holding pressure constant, the
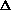 (PV) becomes P
(PV) becomes P
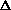 V and cancels with the w = P
V and cancels with the w = P
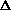 V from
V from
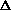 E, giving
E, giving
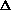 H = q. If pressure is not constant, does that mean
H = q. If pressure is not constant, does that mean
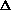 H = q + w +
H = q + w +
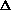 (PV) or
(PV) or
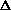 H = q + P
H = q + P
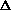 V +
V +
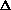 (PV) is true? What does this mean, practically? I just am wondering why are there two "PV" expressions in the first place? What even is
(PV) is true? What does this mean, practically? I just am wondering why are there two "PV" expressions in the first place? What even is
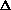 (PV)? It doesn't make sense to me like P
(PV)? It doesn't make sense to me like P
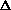 V, what about
V, what about
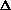 (PV) makes it so it can be added on initially?
(PV) makes it so it can be added on initially?
I know I asked a lot of questions, I'm just trying to make my confusion clear. (Ha)
To:
I'm confused as to why you add the product of the pressure and volume of the system to the internal energy to get enthalpy. Is it just because "that's what enthalpy is defined as"? I think I understand that when holding pressure constant, the
I know I asked a lot of questions, I'm just trying to make my confusion clear. (Ha)
Attachments
-
 delta.gif63 bytes · Views: 2,829
delta.gif63 bytes · Views: 2,829 -
 delta.gif63 bytes · Views: 2,947
delta.gif63 bytes · Views: 2,947 -
 delta.gif63 bytes · Views: 2,858
delta.gif63 bytes · Views: 2,858 -
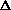 delta.gif63 bytes · Views: 2,916
delta.gif63 bytes · Views: 2,916 -
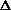 delta.gif63 bytes · Views: 2,747
delta.gif63 bytes · Views: 2,747 -
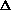 delta.gif63 bytes · Views: 2,663
delta.gif63 bytes · Views: 2,663 -
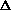 delta.gif63 bytes · Views: 2,551
delta.gif63 bytes · Views: 2,551 -
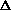 delta.gif63 bytes · Views: 2,654
delta.gif63 bytes · Views: 2,654 -
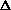 delta.gif63 bytes · Views: 2,569
delta.gif63 bytes · Views: 2,569 -
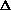 delta.gif63 bytes · Views: 2,648
delta.gif63 bytes · Views: 2,648 -
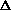 delta.gif63 bytes · Views: 2,644
delta.gif63 bytes · Views: 2,644 -
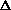 delta.gif63 bytes · Views: 2,495
delta.gif63 bytes · Views: 2,495 -
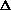 delta.gif63 bytes · Views: 2,640
delta.gif63 bytes · Views: 2,640 -
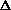 delta.gif63 bytes · Views: 2,622
delta.gif63 bytes · Views: 2,622 -
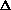 delta.gif63 bytes · Views: 2,556
delta.gif63 bytes · Views: 2,556 -
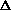 delta.gif63 bytes · Views: 2,604
delta.gif63 bytes · Views: 2,604 -
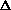 delta.gif63 bytes · Views: 2,622
delta.gif63 bytes · Views: 2,622