Discussion Overview
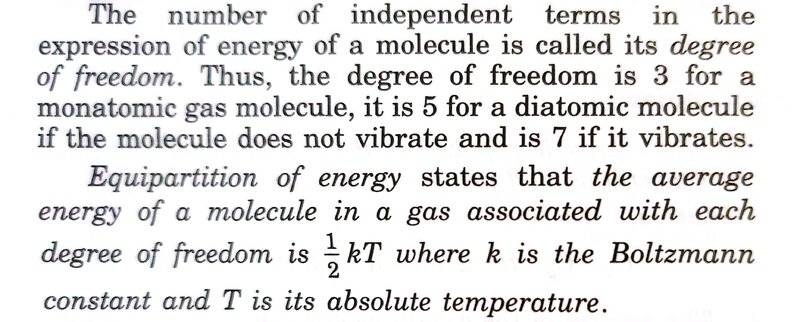
The discussion revolves around the conflicting definitions of "degree of freedom" in the context of the Kinetic Theory of Gases, particularly focusing on how it relates to the energy of gas molecules. Participants explore the implications of these definitions in relation to the equipartition theorem and the characterization of monatomic and diatomic gas molecules.
Discussion Character
- Debate/contested
- Conceptual clarification
- Technical explanation
Main Points Raised
- Some participants note a conflict between the definition of degree of freedom as the number of independent variables and the equipartition theorem's implication that it refers to any one of those independent variables.
- One participant suggests that a monatomic gas has three degrees of freedom and a diatomic gas has five, linking this to the average energy per degree of freedom as described by the equipartition theorem.
- Another participant criticizes the textbook's formulation as inaccurate and misleading, recommending an alternative source for clarity.
- A later reply emphasizes that the equipartition theorem applies to phase-space degrees of freedom that enter the Hamiltonian quadratically, detailing how this affects the mean energy contributions for different types of gas molecules.
Areas of Agreement / Disagreement
Participants express disagreement regarding the definitions of degree of freedom, with some asserting that the textbook is misleading while others attempt to clarify the concepts involved. No consensus is reached on a single definition or interpretation.
Contextual Notes
There are unresolved assumptions regarding the definitions of terms and the implications of the equipartition theorem, as well as the conditions under which different degrees of freedom apply to various types of gas molecules.