tomtomtom1
- 160
- 8
- TL;DR
- Difference between Absolute and Gauge Pressure.
Hello all
I was wondering someone could help clear up my understanding about the difference between Absolute and Gauge Pressure.
After some reading i have been told that the Absolute Pressure is pressure taken at 0 relative to a vacuum.
I am trying to understand what this actually means.
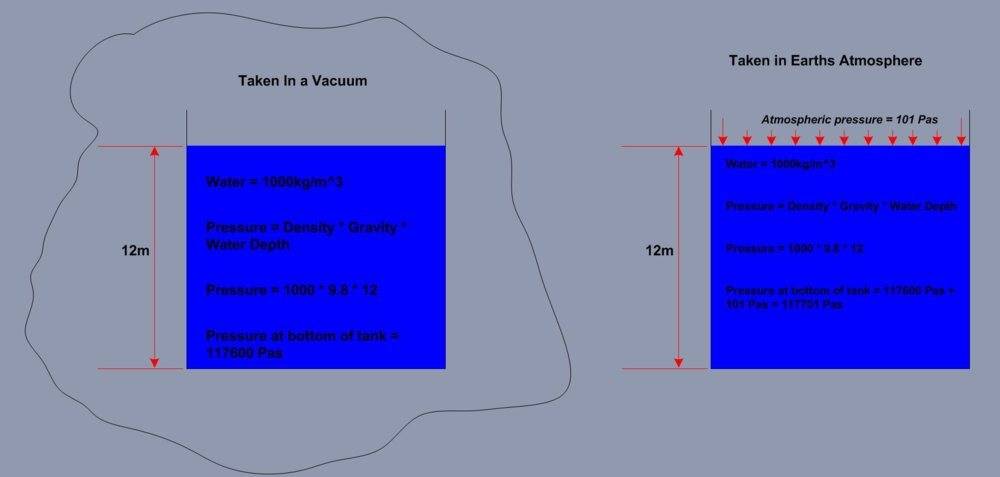
Below is a sketch i made where i have a tank of water for which i calculated the pressure at the bottom of the tank, this tank is in a Vaccum.
I calculated the same pressure on Earth with the same formula but added the Atmospheric pressure.
But i still don't get it?
Can someone help?
Thank you.
I was wondering someone could help clear up my understanding about the difference between Absolute and Gauge Pressure.
After some reading i have been told that the Absolute Pressure is pressure taken at 0 relative to a vacuum.
I am trying to understand what this actually means.
Below is a sketch i made where i have a tank of water for which i calculated the pressure at the bottom of the tank, this tank is in a Vaccum.
I calculated the same pressure on Earth with the same formula but added the Atmospheric pressure.
But i still don't get it?
Can someone help?
Thank you.