Archduke
- 59
- 1
Homework Statement
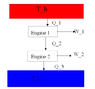
There are two Carnot engines in series, so that the heat exhaust from the first engine drives the second. Find the overall efficiency of the arrangement which contains only the efficiences of the individual engines.
Homework Equations
\epsilon = \frac{W}{Q_{input}}
The Attempt at a Solution
OK, I thought this was really easy, but it just doesn't seem right.
\epsilon = \frac{W_{1}+W_{2}}{Q_{1}} = \frac{(Q_{1} - Q_{2}) + (Q_{2} - Q_{3})}{Q_{1}} = 1 - \frac{Q_{3}}{Q_{1}}
But, surely that's saying that if you put two carnots in series that you get improved efficiency (As Q_{3} < Q_{2}), so if you put an infinite amount of carnot engines in series, you'll be able to have a 100% efficient heat engine!
So, I've either cured the world's energy problems