- #1
Aakash Sunkari
- 13
- 1
- TL;DR Summary
- How does one go about calculating the energy a particle needs such that in a collision with a bound proton, the proton is converted to a neutron?
Hey everyone,
I've got a question on converting bound protons into neutrons.
a. What are some methods used to achieve the proton-to-neutron conversion in atomic nuclei?
I'm familiar with particle scattering off a proton in the nucleus. I'm also aware of (n,p) reactions. Are there any other methods to either convert a proton to a neutron in a nucleus, or any other methods of replacing a proton with a neutron?
Note that I don't mean natural processes such at beta-plus decay.
b. How does one calculate (in the scattering scenario) the amount of energy a particle needs in order to convert a bound proton to a neutron?
Specifically, I am looking at electron-proton collisions in a completely ionized 48Ti isotope:
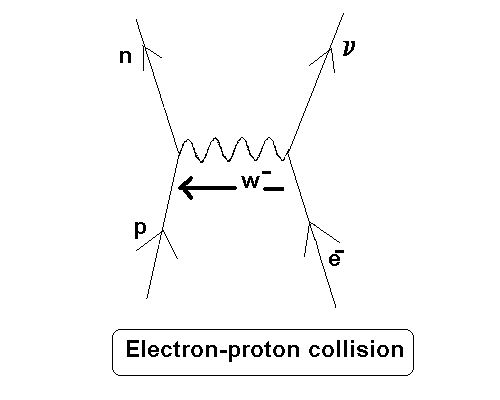
How would one calculate the amount of energy the electron needs to create that proton-to-neutron conversion in the nucleus?
Thank you all in advance!
I've got a question on converting bound protons into neutrons.
a. What are some methods used to achieve the proton-to-neutron conversion in atomic nuclei?
I'm familiar with particle scattering off a proton in the nucleus. I'm also aware of (n,p) reactions. Are there any other methods to either convert a proton to a neutron in a nucleus, or any other methods of replacing a proton with a neutron?
Note that I don't mean natural processes such at beta-plus decay.
b. How does one calculate (in the scattering scenario) the amount of energy a particle needs in order to convert a bound proton to a neutron?
Specifically, I am looking at electron-proton collisions in a completely ionized 48Ti isotope:
How would one calculate the amount of energy the electron needs to create that proton-to-neutron conversion in the nucleus?
Thank you all in advance!