JoJoQuinoa
- 17
- 0
Hello,
I was wondering if someone could show me how to determine the number of orbitals available for a state and the number of electrons in that state. For calcium in the ground state, the electron config is 1s2 2s2 2p6 3s2 3p6 4s2. For the first excited state I assumed 1s2... 4s1 3d1.
From the solution for g_g, why the number of available orbitals is one when 4s is filled? Shouldn't it be zero?
Thanks!
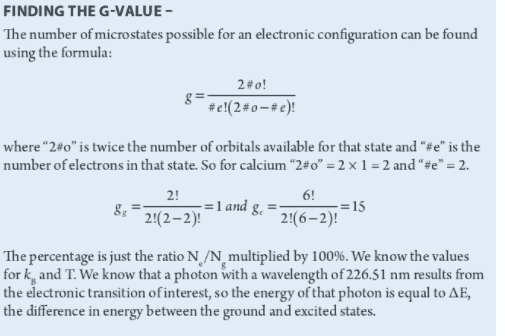
I was wondering if someone could show me how to determine the number of orbitals available for a state and the number of electrons in that state. For calcium in the ground state, the electron config is 1s2 2s2 2p6 3s2 3p6 4s2. For the first excited state I assumed 1s2... 4s1 3d1.
From the solution for g_g, why the number of available orbitals is one when 4s is filled? Shouldn't it be zero?
Thanks!