lorenz0
- 151
- 28
- Homework Statement
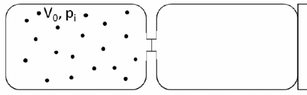
- Two boxes of equal volume ##V_0## are separated by a membrane. The first one to the left, thermally isolated contains ##n## moles of perfect biatomic gas at pressure ##p_i## and temperature ##T_i##. The second box is empty, and in contact with a source of heat at a temperature which is ##T_i/2##. At a certain instant the membrane is removed and the gas reaches a new equilibrium. Find: a) the final pressure of the gas ##P_f## b) the heat ##Q## exchanged with the source of heat c) the variation of entropy of the universe ##\Delta U_{univ}##
- Relevant Equations
- ##PV=nRT, \Delta U=nC_V \Delta T=Q-L, \Delta S=nC_V \ln(\frac{T_f}{T_i})+nR\ln(\frac{V_f}{V_i})##
a) ##P_f=\frac{nRT_f}{V_f}=\frac{nR\frac{T_i}{2}}{2V_0}=\frac{1}{4}\frac{nRT_i}{V_0}=\frac{1}{4}P_i##
b) ##Q=\Delta U=nC_V \Delta T=n\frac{5}{2}R(-\frac{T_i}{2})=-\frac{5}{4}nRT_i=-\frac{5}{4}P_i V_0## (##L=0## since the gas expands in a vacuum;Now, (a) and (b) are both correct but not (c), for which I get:c) ##\Delta S_{system}=nC_V \ln(\frac{T_f}{T_i})+nR\ln(\frac{V_f}{V_i})=n\frac{5}{2}R\ln(\frac{\frac{T_i}{2}}{T_i})+nR\ln(\frac{2V_0}{V_0})=-\frac{3}{2}nR\ln(2)## I understand that the entropy of the system decreases since heat goes out of the system so I guessed that the entropy of the universe should go up by the same amount but apparently this is wrong as in the solution given in the text is ##n\frac{5}{2}R-\frac{3}{2}nR\ln(2)##. What is it that I am missing in finding the entropy of the universe? How should I reason about such a problem in general? Thanks
b) ##Q=\Delta U=nC_V \Delta T=n\frac{5}{2}R(-\frac{T_i}{2})=-\frac{5}{4}nRT_i=-\frac{5}{4}P_i V_0## (##L=0## since the gas expands in a vacuum;Now, (a) and (b) are both correct but not (c), for which I get:c) ##\Delta S_{system}=nC_V \ln(\frac{T_f}{T_i})+nR\ln(\frac{V_f}{V_i})=n\frac{5}{2}R\ln(\frac{\frac{T_i}{2}}{T_i})+nR\ln(\frac{2V_0}{V_0})=-\frac{3}{2}nR\ln(2)## I understand that the entropy of the system decreases since heat goes out of the system so I guessed that the entropy of the universe should go up by the same amount but apparently this is wrong as in the solution given in the text is ##n\frac{5}{2}R-\frac{3}{2}nR\ln(2)##. What is it that I am missing in finding the entropy of the universe? How should I reason about such a problem in general? Thanks