PhoenixWright
- 20
- 1
Hello.
I have found an exercise that reads: "The wave function of a one-electron atom is:
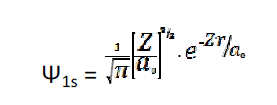 [1s orbital, atom: Li2+ What will be the most likely distance to locate the electron?"
[1s orbital, atom: Li2+ What will be the most likely distance to locate the electron?"
Solution of exercise is:
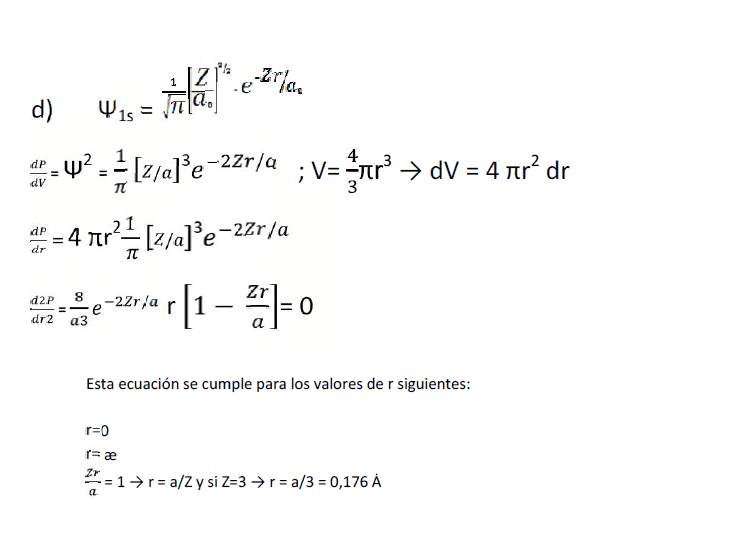
My question is: Why the square of the probability density is dP/dV, and therefore the volume formula is used?
I had always done this exercise by the square of the wave function, deriving the function and finding the maximum... I don't know why it's used the volume of a sphere here...
Thank you!
I have found an exercise that reads: "The wave function of a one-electron atom is:
Solution of exercise is:
My question is: Why the square of the probability density is dP/dV, and therefore the volume formula is used?
I had always done this exercise by the square of the wave function, deriving the function and finding the maximum... I don't know why it's used the volume of a sphere here...
Thank you!