Klacid
- 2
- 0
Hey guys i wanted to know how make some chemicals when i have an equation, for example:
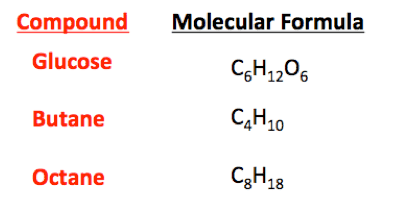
This discussion focuses on the process of creating chemicals from molecular formulas and chemical equations. It emphasizes that molecular formulas represent compounds and that a typical chemical reaction is expressed as A + B → C + D. The conversation highlights the importance of understanding chemical reactions, safety precautions, and the necessity of having the correct precursors for successful chemical synthesis. Additionally, it confirms that learning chemistry enables individuals to derive equations from molecular formulas, although the feasibility of reactions must be established beforehand.
PREREQUISITESChemistry students, laboratory technicians, and anyone interested in chemical synthesis and reaction mechanisms will benefit from this discussion.
First of all, you don't have any equations in the graphic above. These are called the molecular formulas for the various compounds shown.Klacid said:Hey guys i wanted to know how make some chemicals when i have an equation, for example:
