- #1
blaisem
- 28
- 2
Looking for a practical description. I have a feeling I am grossly misunderstanding something fundamental about electric circuits and apologize if my questions are confusing because of it. I have these two sources:
acs
hyperphysics
My (probably naive) understanding of the process is as follows:
The acs source says in the very last sentence:
"If you connect the n-type and p-type layers with a metallic wire, the electrons will travel from the n-type layer to the p-type layer by crossing the depletion zone and then go through the external wire back of the n-type layer, creating a flow of electricity."
This reads to me like the electrons "double back." They go from n -> p -> n -> copper wire. I thought a circuit needed to flow in one direction. In fact, in order for the copper wire to attract n-type electrons, the copper wire end attached to the n-type must be positively charged like an anode. However, that would mean the cathode/anode ends of the copper wire would be oppositely charged to the dipole orientation at the junction. As I understand, this would constitute a reverse-bias circuit, working against the chemical potential of diffusion by acting against the accumulation of an opposing electric potential within:
Reverse-Bias Circuit
I would think the potential should be in the opposite direction and consistent with the charge distribution of the depletion region. Electrons should flow from the negative p-type into the copper wire's anode, around the wire to the cathode, and into the positively charged n-type, replacing electrons at the depletion zone that had moved into holes resulting from outflowing electrons in the p-type. That would be a forward-biased circuit as shown:
Forward-Bias Circuit

This however contradicts the ACS source which explicitly depicts that in a solar cell, the electrons flow from the n-type into the wire and reenter at the p-type, as seen in the top right of this diagram:
Solar Cell Circuit

1.
Could someone please explain what are the correct electric signs at each end of the copper wire, and how these come to be? My goal is to understand how electrons flow from the n-type into the copper wire and back into the p-type after electronic photoexcitation, and why this can proceed in the opposite direction of the depletion zone's dipole. I don't understand how the cathode/anode ends of the copper wire can coexist with an oppositely oriented dipole in the depletion field. I expect the two electric potentials to work in opposition.
2.
Why is sunlight even necessary? If the chemical potential of a pn-junction is already sufficient to drive the formation of a potential, can't we just attach a copper wire, whose electrons are driven by the potential from diffusion, and already have a functioning circuit?
Thank you for your time.
acs
hyperphysics
My (probably naive) understanding of the process is as follows:
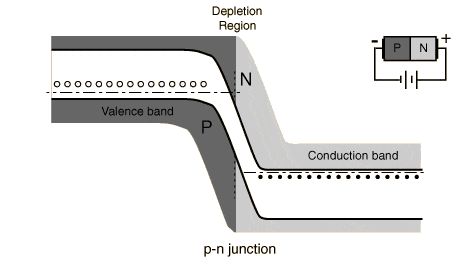
- p-type has no free electrons; n-type has free electrons.
- chemical potential drives n-type electrons to diffuse into the p-type region, leaving the n-type semiconductor positive and the p-type semiconductor negative, forming a dipole between the two types across a region over the junction called the depletion zone.
- When the electric potential of the dipole is equal to the chemical potential driving diffusion, diffusion stops and the system is in electrical/entropic equilibrium.
- Sunlight excites electrons in the p-type semiconductor. These electrons move to the LUMO of the n-type semiconductor (conduction band), because the band gap has been decreased across the two semiconductors as a result of the diffusion. The p-type's homo has been increased in energy as a result of the negative charge in its valence band, while the lumo of the n-type has been decreased in energy as a result of the positive charge in its valence band.
- Once the p-type valence band electron has been excited into the n-type conduction band, it moves to a copper wire to generate current? Here's where I'm stuck.
The acs source says in the very last sentence:
"If you connect the n-type and p-type layers with a metallic wire, the electrons will travel from the n-type layer to the p-type layer by crossing the depletion zone and then go through the external wire back of the n-type layer, creating a flow of electricity."
This reads to me like the electrons "double back." They go from n -> p -> n -> copper wire. I thought a circuit needed to flow in one direction. In fact, in order for the copper wire to attract n-type electrons, the copper wire end attached to the n-type must be positively charged like an anode. However, that would mean the cathode/anode ends of the copper wire would be oppositely charged to the dipole orientation at the junction. As I understand, this would constitute a reverse-bias circuit, working against the chemical potential of diffusion by acting against the accumulation of an opposing electric potential within:
Reverse-Bias Circuit
I would think the potential should be in the opposite direction and consistent with the charge distribution of the depletion region. Electrons should flow from the negative p-type into the copper wire's anode, around the wire to the cathode, and into the positively charged n-type, replacing electrons at the depletion zone that had moved into holes resulting from outflowing electrons in the p-type. That would be a forward-biased circuit as shown:
Forward-Bias Circuit
This however contradicts the ACS source which explicitly depicts that in a solar cell, the electrons flow from the n-type into the wire and reenter at the p-type, as seen in the top right of this diagram:
Solar Cell Circuit
1.
Could someone please explain what are the correct electric signs at each end of the copper wire, and how these come to be? My goal is to understand how electrons flow from the n-type into the copper wire and back into the p-type after electronic photoexcitation, and why this can proceed in the opposite direction of the depletion zone's dipole. I don't understand how the cathode/anode ends of the copper wire can coexist with an oppositely oriented dipole in the depletion field. I expect the two electric potentials to work in opposition.
2.
Why is sunlight even necessary? If the chemical potential of a pn-junction is already sufficient to drive the formation of a potential, can't we just attach a copper wire, whose electrons are driven by the potential from diffusion, and already have a functioning circuit?
Thank you for your time.