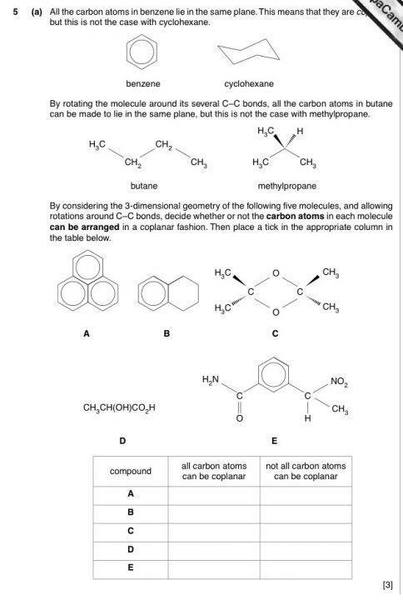
SUMMARY
This discussion focuses on determining the coplanarity of carbon atoms in various molecular structures, specifically in compounds C, D, and E. Participants clarify that while sp3 hybridized carbon atoms typically adopt a tetrahedral geometry, this does not inherently prevent them from being coplanar under certain arrangements. The conversation highlights that in structures like methylpropane, although any three carbon atoms can be coplanar, the fourth carbon atom disrupts this arrangement. The importance of visual models for understanding molecular geometry is emphasized throughout the discussion.
PREREQUISITES
- Understanding of sp3 and sp2 hybridization
- Familiarity with molecular geometry and bond angles
- Basic knowledge of molecular models and modeling techniques
- Experience with structural representations of organic compounds
NEXT STEPS
- Explore molecular modeling software such as ChemDraw or Avogadro
- Study the principles of hybridization in organic chemistry
- Learn about the geometric arrangements of carbon atoms in various hydrocarbons
- Investigate the effects of molecular rigidity on coplanarity in cyclic compounds
USEFUL FOR
Chemistry students, organic chemists, educators teaching molecular geometry, and anyone interested in understanding the spatial arrangements of carbon atoms in organic compounds.