- #1
Janiceleong26
- 276
- 4
1. Homework Statement
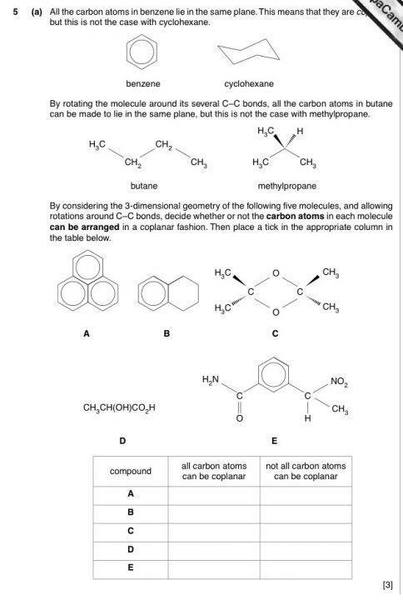
I can see that in B, not all C atoms are coplanar , but for C, D and E I can't see how the carbon atoms are coplanar .. I thought they are tetrahedral?
Homework Equations
The Attempt at a Solution
I can see that in B, not all C atoms are coplanar , but for C, D and E I can't see how the carbon atoms are coplanar .. I thought they are tetrahedral?