- #1
Rzbs
- 52
- 11
- TL;DR Summary
- Importance of energy gap in electronic transport properties
In the solid state physics by Ashcroft & Mermin, in chapter 9 there is a paragraph that I would be grateful if anyone could explain it more for me. The paragraph is:
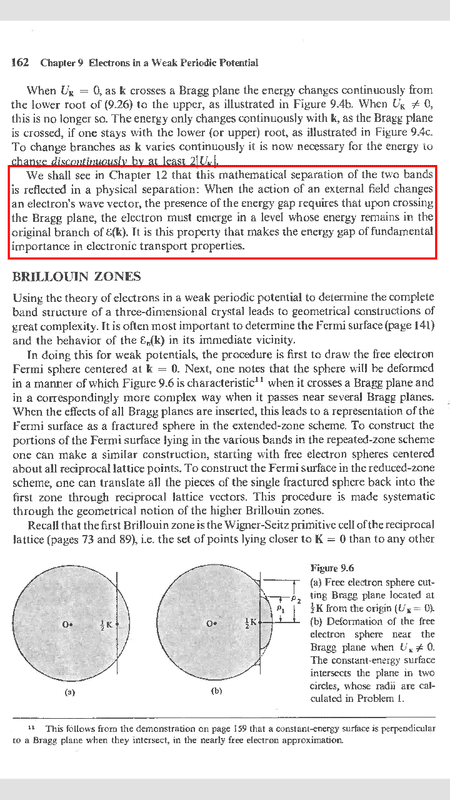
As it said in chapter 12 it will be seen. I read chapter 12 but unfortunately I can't understand what exactly it want to say...
As it said in chapter 12 it will be seen. I read chapter 12 but unfortunately I can't understand what exactly it want to say...
Last edited by a moderator: