- 23,721
- 5,934
- TL;DR
- Thermodynamics Learning Problem
I came across this very interesting Thermodynamics problem in PhysicsStackExchange. It was deleted by the OP because the moderators, in their infinite wisdom, gave him a hard time about its being a homework problem which was, in their opinion, a "check my work" post, rather than a "I'm having difficulty solving this" post. In the end, he became frustrated, received no help, and deleted his thread. Here is the problem statement. His difficulty was with part b, which should be our focus.
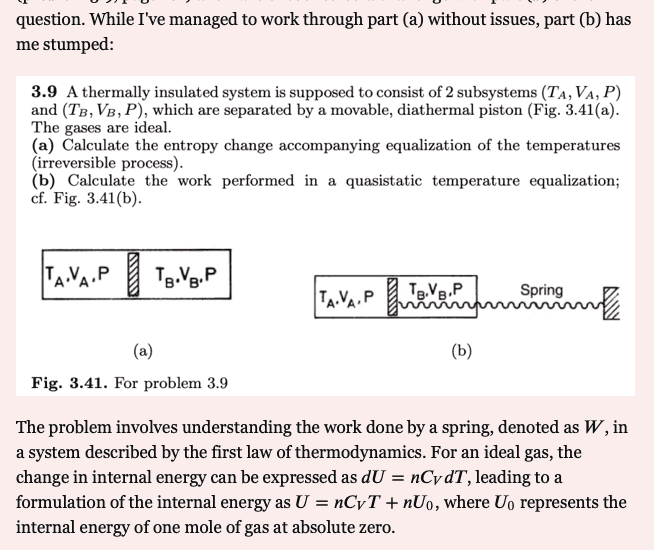
I personally had difficulty correctly analyzing this, and it took me about a day to finally reason it out. In my judgment, this problem would be worth considering by PF members studying Thermodynamics and others as well. Maybe even the guy who originally submitted it to StackExchange might come across this, and participate.
I am inviting PF members to participate in discussing and solving this problem. Again, the focus is part b.
I personally had difficulty correctly analyzing this, and it took me about a day to finally reason it out. In my judgment, this problem would be worth considering by PF members studying Thermodynamics and others as well. Maybe even the guy who originally submitted it to StackExchange might come across this, and participate.
I am inviting PF members to participate in discussing and solving this problem. Again, the focus is part b.