- #1
Keru
- 20
- 1
Homework Statement
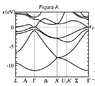
The exercise asks many questions about the following E(k) diagram, but I'm more interested in understanding some basic things about it, from which I'm sure i'll be able to find the answers I'm requested.
1) What do the bands actually correspond to? Is the lowest band corresponding to 1s2 molecular orbital, the upper to 2s2 2p6 and so on?
2) I have several diagrams and all of them contain a "Γ", what is the meaning of that 'k' apparently common to every band diagram?
3) Looking at the Fermi level, would this statements be true?
3.1) At 0K the first 4 bands would be full, and the rest empty.
3.2) At a little higher temperature (let's say 0,1eV more for the Fermi level), the first three bands would be full, while the next two partially filled.
4) If 1) was correct, by looking at the intersection of Fermi level and Γ, the graphic could only correspond to the semimetals Ge or As, right?
Thanks in advantage for any help.