Astrocyte
- 15
- 4
- Homework Statement
- What is the change in entropy of van der Waals gas in free expansion?
Free expansion from V to 2V
- Relevant Equations
- (p+aN^2/V^2)(V-Nb)=NkT
Previous of this problem, there was another problem. that is "What is the change in Temperature of van der Waals gas in free expansion?".
I got them.
It was
C_V dT= -aN^2/V^2 dV
Then, I got
T=T0-aN^2/2VC_V
So i knew that the Temperature is decreased by free expansion in adiabatic process.
Then I calculated some about the change of entropy.
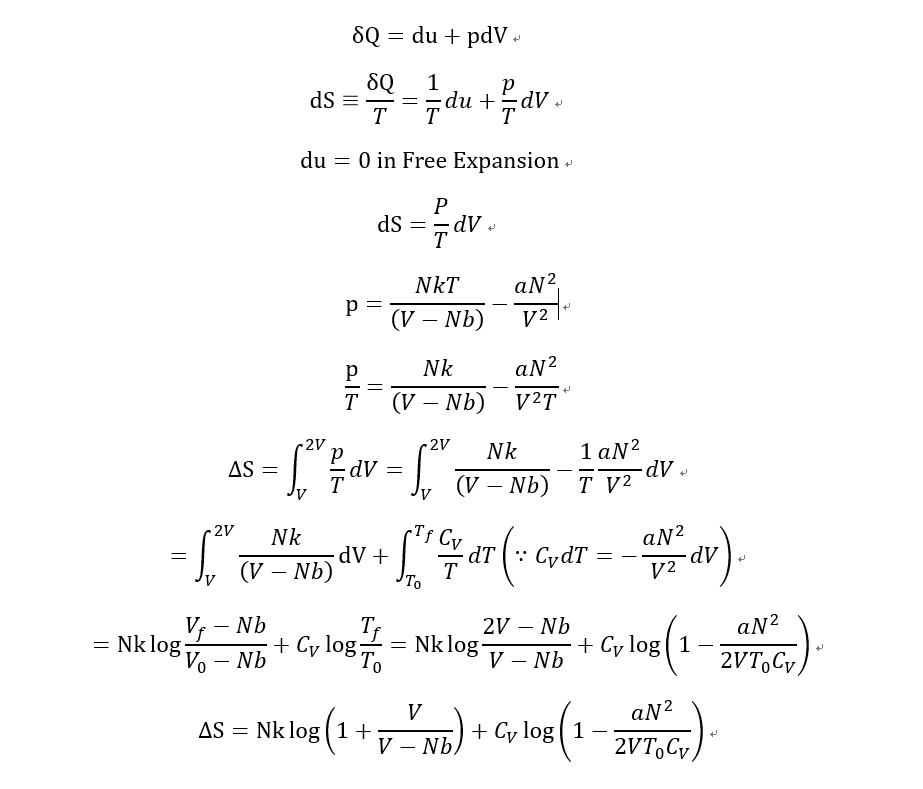
And I notice that this result says "entropy can be decreased for Free expansion of Waals gas.
What did i do wrong for this calculation?
I got them.
It was
C_V dT= -aN^2/V^2 dV
Then, I got
T=T0-aN^2/2VC_V
So i knew that the Temperature is decreased by free expansion in adiabatic process.
Then I calculated some about the change of entropy.
And I notice that this result says "entropy can be decreased for Free expansion of Waals gas.
What did i do wrong for this calculation?
Last edited: