SUMMARY
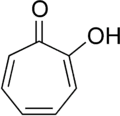
Tropolone is confirmed as an aromatic compound due to its adherence to Hückel's rule, possessing 6 π electrons in a conjugated, cyclic system. The discussion clarifies that the carbon atom connected to the oxygen via a double bond does indeed have a p-orbital, contributing to the overall aromaticity. Experimental validation can be achieved through techniques such as NMR spectroscopy, which can provide insights into the compound's electronic structure. The confusion surrounding the presence of p-orbitals in carbonyl groups is addressed, emphasizing the importance of understanding pi bonds in aromatic systems.
PREREQUISITES
- Understanding of Hückel's rule for aromaticity
- Knowledge of pi bonds and p-orbitals
- Familiarity with NMR spectroscopy techniques
- Basic concepts of resonance in organic chemistry
NEXT STEPS
- Research the application of Hückel's rule in different aromatic compounds
- Learn about the principles of NMR spectroscopy and its use in determining aromaticity
- Explore the concept of resonance and its implications in organic structures
- Study the characteristics of various aromatic compounds beyond Tropolone
USEFUL FOR
Chemistry students, organic chemists, and researchers interested in the properties of aromatic compounds and their electronic structures.